Tropomyosin: Difference between revisions
No edit summary |
No edit summary |
||
| (7 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
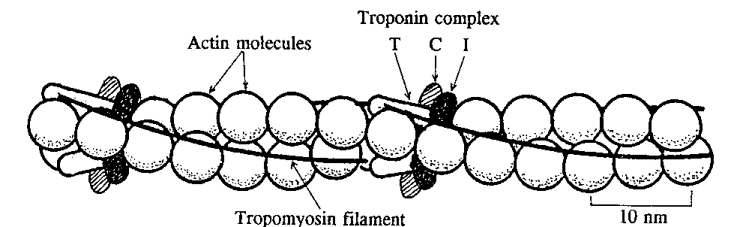
< | According to B. Alberts<ref>B. Alberts, Molecular Biology of the Cell, Garland Science, 5th edition, 2008</ref> tropomyosin is an elongated [[Protein|protein]], which stabilizes [[Actin filaments|actin filaments]] by binding to seven adjacent [[Actin|actin]] subunits, thus preventing it from interacting with other proteins <ref>B. Alberts, Molecular Biology of the Cell, Garland Science, 5th edition, 2008</ref>. Tropomyosin has a coiled coil structure caused by the joining of two alpha helical monomers <ref>Regulatory Proteins, (n.d.), [Online], Available: http://www.uic.edu/classes/phyb/phyb516/regulatoryproteinsu3.htm [27 November 2013]</ref>. Tropomyosin, along with [[Troponin|troponin]] play an important role in regulation of [[Muscle|muscle]] contraction <ref>R.D Keynes, D.J Aidley, Nerve and Muscle, 3rd edition, Cambridge University Press, 2001</ref>. As tropomyosin binds to actin it follows its helical structure <ref>B.Alberts, Molecular Biology of the Cell, 5th edition, Garland Science, 2008</ref>. Fig.1 | ||
<references / | |||
[[Image:Tropomyosin.gif|Accessory proteins troponin and tropomyosin on the actin filament]]<ref>San Diego State University (unknown), Skeletal Muscle Structure and Function, http://www-rohan.sdsu.edu/course/ens304/public_html/section1/Muscle.htm</ref> | |||
This figure describes the relative position of accesory proteins and [[Actin filaments|actin]] in a thin filament. Troponin complex is bound to actin and tropomyosin, which lies in the groove of the actin helix. | |||
'''Contraction''' | |||
When a muscle is relaxed, tropomyosin is blocking the myosin binding sites on the thin filament. During the process of contraction, Ca2+ molecules bind to the C-subunit on Troponin which causes the molecule to change its structure, this then pulls away from the myosin binding site and brings tropomyosin a long with it. This then reveals the binding site and allows for the binding of myosin to the thin filament and the beginning of contraction. | |||
=== References === | |||
<references /> | |||
Latest revision as of 05:15, 29 November 2013
According to B. Alberts[1] tropomyosin is an elongated protein, which stabilizes actin filaments by binding to seven adjacent actin subunits, thus preventing it from interacting with other proteins [2]. Tropomyosin has a coiled coil structure caused by the joining of two alpha helical monomers [3]. Tropomyosin, along with troponin play an important role in regulation of muscle contraction [4]. As tropomyosin binds to actin it follows its helical structure [5]. Fig.1
This figure describes the relative position of accesory proteins and actin in a thin filament. Troponin complex is bound to actin and tropomyosin, which lies in the groove of the actin helix.
Contraction
When a muscle is relaxed, tropomyosin is blocking the myosin binding sites on the thin filament. During the process of contraction, Ca2+ molecules bind to the C-subunit on Troponin which causes the molecule to change its structure, this then pulls away from the myosin binding site and brings tropomyosin a long with it. This then reveals the binding site and allows for the binding of myosin to the thin filament and the beginning of contraction.
References
- ↑ B. Alberts, Molecular Biology of the Cell, Garland Science, 5th edition, 2008
- ↑ B. Alberts, Molecular Biology of the Cell, Garland Science, 5th edition, 2008
- ↑ Regulatory Proteins, (n.d.), [Online], Available: http://www.uic.edu/classes/phyb/phyb516/regulatoryproteinsu3.htm [27 November 2013]
- ↑ R.D Keynes, D.J Aidley, Nerve and Muscle, 3rd edition, Cambridge University Press, 2001
- ↑ B.Alberts, Molecular Biology of the Cell, 5th edition, Garland Science, 2008
- ↑ San Diego State University (unknown), Skeletal Muscle Structure and Function, http://www-rohan.sdsu.edu/course/ens304/public_html/section1/Muscle.htm