Proline: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
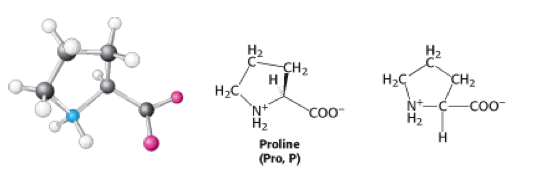
Proline is an [[Imino acid|imino acid]]. It has a molecular weight of 115.13 and its molecular formula is C<sub>5</sub>H<sub>9</sub>NO<sub>2</sub>.<sub></sub> It is also classified as a hydrophobic amino acid.<br> | |||
<sub></sub>Proline has an [[Aliphatic|aliphatic]] side chain, which is bonded to the nitrogen atom and the [[Alpha-carbon|alpha-carbon]] [[Atom|atom]]. It influences [[Protein|protein]] architecture, because it's structure makes it more conformationally restricted than other [[Amino acids|amino acids]] <ref>Biochemistry 6th ed. 2006, J.Berg et al</ref>. | |||
<sub></sub>Proline has an [[Aliphatic|aliphatic]] side chain, which is bonded to the nitrogen atom and the [[Alpha-carbon|alpha-carbon]] [[Atom|atom]]. It influences [[Protein|protein]] architecture, because it's structure makes it more conformationally restricted than other [[Amino acids|amino acids]] <ref>Biochemistry 6th ed. 2006, J.Berg et al</ref>. | |||
[[Image:Proline.png]] | [[Image:Proline.png]] | ||
Revision as of 08:31, 10 October 2014
Proline is an imino acid. It has a molecular weight of 115.13 and its molecular formula is C5H9NO2. It is also classified as a hydrophobic amino acid.
Proline has an aliphatic side chain, which is bonded to the nitrogen atom and the alpha-carbon atom. It influences protein architecture, because it's structure makes it more conformationally restricted than other amino acids [1].
References
- ↑ Biochemistry 6th ed. 2006, J.Berg et al