Optical isomers: Difference between revisions
Jump to navigation
Jump to search
Added a picture |
No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
[[Image: | [[Image:Optical isomerism.GIF|right|Optical isomers are non-superimposable]] | ||
Optical isomers rotate the plane of plane polarised light by 90° clockwise or anticlockwise. Optical isomers will have a central, chiral carbon atom. Chirality depends on the central C atom having four different groups attached to it as this makes the molecule non-superimposabe on its mirror image (asymmetrical)<ref>This is a reference to fckLRhttp://www.chemguide.co.uk/basicorg/isomerism/optical.html</ref>. Optical isomers (enantiomers) rotate the plane of plane polarised light in opposite directions. | Optical isomers rotate the plane of plane polarised light by 90° clockwise or anticlockwise. Optical isomers will have a central, chiral carbon atom. Chirality depends on the central C atom having four different groups attached to it as this makes the molecule non-superimposabe on its mirror image (asymmetrical)<ref>This is a reference to fckLRhttp://www.chemguide.co.uk/basicorg/isomerism/optical.html</ref>. Optical isomers (enantiomers) rotate the plane of plane polarised light in opposite directions. | ||
Latest revision as of 20:32, 4 December 2016
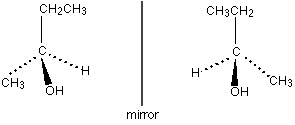
Optical isomers rotate the plane of plane polarised light by 90° clockwise or anticlockwise. Optical isomers will have a central, chiral carbon atom. Chirality depends on the central C atom having four different groups attached to it as this makes the molecule non-superimposabe on its mirror image (asymmetrical)[1]. Optical isomers (enantiomers) rotate the plane of plane polarised light in opposite directions.
Two enantiomers of an optical isomer will have very similar physical and chemical properties making it very hard to tell the difference between enantiomers.
References
- ↑ This is a reference to fckLRhttp://www.chemguide.co.uk/basicorg/isomerism/optical.html