Glycosidic bond: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
Glycosidic bonds join monosaccharides or longer [[Sugar|sugar]] chains to other carbohydrates, forming disaccharides, oligosaccharides and polysaccharides. It is a type of [[Covalent bond|covalent bond]]. | Glycosidic bonds join [[monosaccharides|monosaccharides]] or longer [[Sugar|sugar]] chains to other carbohydrates, forming [[disaccharide|disaccharides]], [[Oligosaccharides|oligosaccharides]] and [[polysaccharide|polysaccharides]]. It is a type of [[Covalent bond|covalent bond]]. | ||
These sugar chains of monosaccharides are able to form further glycosidic bonds with [[Alcohols|alcohols]] and [[Amines|amines]] to produce sugar acetals/glycosides and nucleosides.If the anomeric carbon of the sugar forms the bond with the oxygen atom in the hydroxyl group in the alcohol, the bond is named an O-glycosidic bond. Conversely, if the anomeric carbon of the sugar forms the bond with the nitrogen atom of an amine, the bond is then called a N-glycosidic bond | These sugar chains of [[monosaccharides|monosaccharides]] are able to form further glycosidic bonds with [[Alcohols|alcohols]] and [[Amines|amines]] to produce sugar acetals/glycosides and nucleosides. If the anomeric carbon of the sugar forms the bond with the [[oxygen|oxygen]] atom in the [[hydroxyl group|hydroxyl group]] in the alcohol, the bond is named an O-glycosidic bond. Conversely, if the anomeric carbon of the sugar forms the bond with the [[nitrogen|nitrogen]] atom of an [[amine|amine]], the bond is then called a N-glycosidic bond <ref>'Biochemistry', Sixth Edition, (2006), Jeremy M. Berg, John L. Tymoczko and Lubert Stryer, p.309-310</ref>. | ||
<br> | <br> | ||
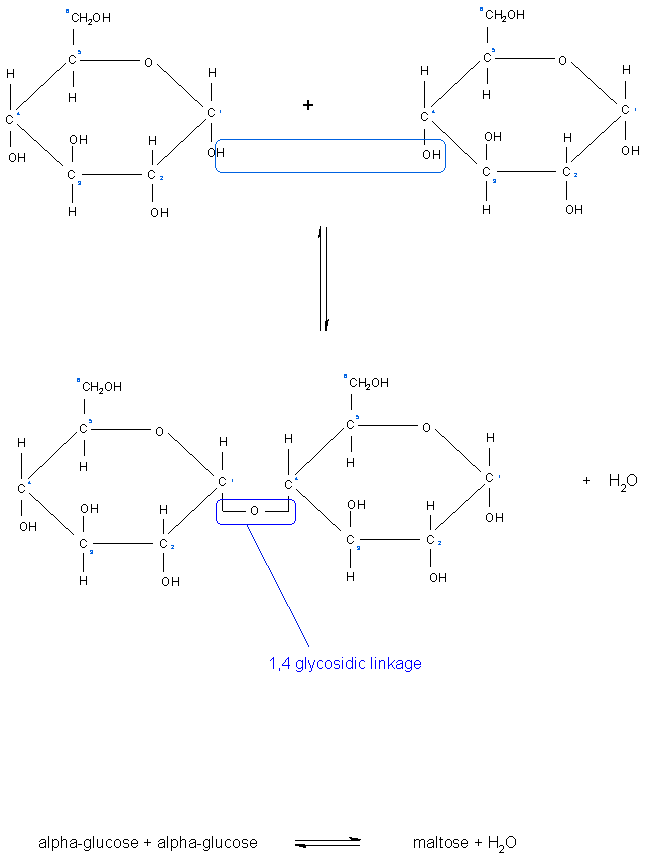
[[Image: | [[Image:Formation of glycosidic bond.gif|Formation of a glycosidic bond between two glucose sugars.]]<ref>http://www.nicksnowden.net/images/Macromolecules/maltose%20formation.GIF - 'Carbohydrates', last accessed on 10/1/11</ref><br> | ||
=== References === | === References === | ||
<references /> | <references /> | ||
Revision as of 17:38, 10 January 2011
Glycosidic bonds join monosaccharides or longer sugar chains to other carbohydrates, forming disaccharides, oligosaccharides and polysaccharides. It is a type of covalent bond.
These sugar chains of monosaccharides are able to form further glycosidic bonds with alcohols and amines to produce sugar acetals/glycosides and nucleosides. If the anomeric carbon of the sugar forms the bond with the oxygen atom in the hydroxyl group in the alcohol, the bond is named an O-glycosidic bond. Conversely, if the anomeric carbon of the sugar forms the bond with the nitrogen atom of an amine, the bond is then called a N-glycosidic bond [1].
References
- ↑ 'Biochemistry', Sixth Edition, (2006), Jeremy M. Berg, John L. Tymoczko and Lubert Stryer, p.309-310
- ↑ http://www.nicksnowden.net/images/Macromolecules/maltose%20formation.GIF - 'Carbohydrates', last accessed on 10/1/11