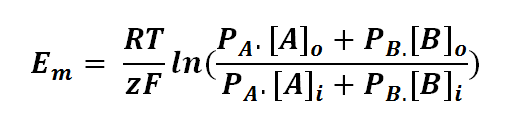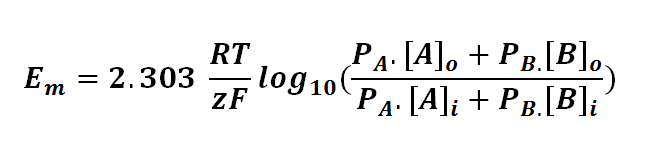Goldman equation: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
'''Goldman equation''' is an equation used to calculate the electrical equilibium potential across the cell's membrane in the presence of more than one ions taking into account the selectivity of membrane's permeability. It is derived from the Nernst equation. <br> | '''Goldman equation''' is an equation used to calculate the electrical equilibium potential across the cell's membrane in the presence of more than one ions taking into account the selectivity of membrane's permeability. It is derived from the Nernst equation. <br> | ||
== Equation<br> == | == Equation<br> == | ||
| Line 33: | Line 33: | ||
[A or B]<sub>o</sub> is the concentration of ion outside the membrane <br> | [A or B]<sub>o</sub> is the concentration of ion outside the membrane <br> | ||
[A or B]<sub>i</sub> is the concentration of ion inside the membrane<br> | [A or B]<sub>i</sub> is the concentration of ion inside the membrane<br> | ||
== See also<br> == | == See also<br> == | ||
*[[Nernst equation]]<br> | *[[Nernst equation]]<br> | ||
== References<br> == | == References<br> == | ||
== External Links == | |||
*[http://www.nernstgoldman.physiology.arizona.edu/ The Nernst/Goldman Equation Simulator] | |||
<br> | <br> | ||
Revision as of 15:32, 15 November 2010
Goldman equation is an equation used to calculate the electrical equilibium potential across the cell's membrane in the presence of more than one ions taking into account the selectivity of membrane's permeability. It is derived from the Nernst equation.
Equation
The Goldman equation can be expressed as follows:
or
where
Em is the potential difference of an ion between membranes
R is the universal gas constant; R = 8.314471 J mol-1
T is the thermodynamics temperature, in Kelvin; 0 K = -273.15oC
z is the number of moles of electrons transferred between membranes (defined by the valency of ion)
F is the Faraday's constant; F = 96,485.3415 C mol-1
PA or B is the permeability of the membrane to a particular ion (A or B)
[A or B]o is the concentration of ion outside the membrane
[A or B]i is the concentration of ion inside the membrane
See also
References
External Links