L-isomer
An L-isomer is a term used in stereochemistry to describe a chemical that has a non superimposable mirror image counterpart. An atom that has four different groups attached to it and gives rise to this phenomenon is called the chiral centre or more commonly the chiral carbon.
The L-isomer:
The L-isomer is the naturally occurring isomer in proteins. The D isomer is man-made. An easy way to remember this is that L stands for 'living'.
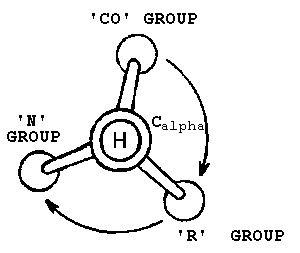
The Corn Law:
The Corn Law can be used to distinguish between the L and the D isomer. For example, in Alanine, the L form of the isomer would only spell the word 'CORN' if you read it counter clockwise from the topmost point.
Nomenclature:
The L stands for levorotary and the D dextrorotary which references their ability to change light polarization.
Example:
An often cited example is the Thalidomide disaster in the 1960s. Thalidomide was used as a anti-sickness medicine in pregnancy however the drug given involved a racemic mixture, one of the isomers was poisonous to unborn foetuses and babies were born with congenital birth defects. The mechanisms of actions are still unknown and debated with over 30 different hypothesis proposed on how thalidomide affects humans and subsequent animal models[1].
Exceptions:
The amino acid Glycine doesnt have a D or an L form because it has 2 hydrogens attached to the alpha carbon. The carbon must be joined to 4 all different groups to be able to undergo D or L isomerisation.
References
1. James H. Kim and Anthony R. Scialli, TOXICOLOGICAL SCIENCES 122(1), 1–6 (2011)
2. Pearson Education Online, The L- and D- isomers.
- ↑ James H. Kim and Anthony R. Scialli, TOXICOLOGICAL SCIENCES 122(1), 1–6 (2011)