Covalent
Covalent bonds are the strongest bonds that join two atoms together. The bonds are formed when an electron pair is shared between the adjacent atoms. The strength of the bond requires high levels of energy to be put in to be broken. Multiple pairs of electrons can be shared to for double or triple covalent bonds.[1]
The term 'covalent', when used within the subject of chemistry, denotes the sharing of an electron by two atoms. The repulsive force the two positive nuclei generate is countered by their mutual attraction for an electron and thus a chemical bond is formed.
Covalent bonding can be contrasted with ionic bonding which entails the electrostatic attraction of two oppositely-charged atoms due to the ‘hijacking’ of electrons by one atom from another rather than a shared 'pool'. This can be seen in compounds such as sodium chloride (Na+Cl-) or magnesium oxide, or magnesia, (Mg2+O2-). This type of bonding confers a greater ability of the compound to dissolve in solution and so ionic compounds display a much higher propensity for dissolution than covalent species.
Covalent bonding is not limited to the sharing of one electron. In the case of a molecule of carbon dioxide (O=C=O) the carbon atom shares two electrons with each oxygen atom which also donates two electrons to the shared pool generating noble gas configuration (a favourable state comprising 8 electrons in the external shell) for each nucleus. In the case of nitrogen gas (N![]() N) the pool is generated by each atom donating 3 electrons, attaining noble gas configuration also.
N) the pool is generated by each atom donating 3 electrons, attaining noble gas configuration also.
Using the idea of quantum electron orbitals, several specific cases of covalent bonding, notably σ-bonding (sigma bonding) and π-bonding (pi-bonding), can occur.
Sigma bonding occurs through the direct overlap of two orbitals along the same bonding plane each containing an electron of opposite spin.
Pi-bonding occurs when two p-orbitals, both perpendicular to the plane of bonding overlap; each containing electrons in opposing spins. Pi-bonding is generally weaker than sigma and only occurs once sigma bonding has already occurred [2][3].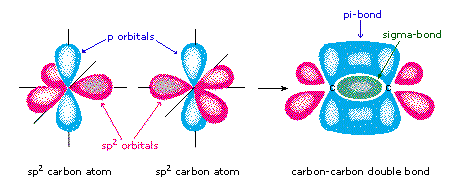
This figure shows the formation of sigma- and pi- bonds through orbital interraction.
A covalent bond is one of three forms of intramolecular forces (covalent, ionic and metallic), and is the strongest type of bonding. This type of bond is formed via the sharing of a pair of electrons on adjacent atoms. Depending on the atoms in which the force acts between, the energy required to break a covalent bond differs. This is due to electronegativity; the more similar the electronegativity of two atoms the stronger the covalent bond. More than one electron pair can be shared to form multiple covalent bonds, for example a double bond or triple bond [4].
Atoms can react together, this allows them to form sometimes very large an complex structures. In order for them to form these large molecuels they must form inter molecular forces or bonds which are strong and stable. A covalent bond occurs between atoms in a concept of "elctron sharing". this involves an atom sharing, or donating, one of it's electrons to another atom. This is formed using a covalent bond, the molecuel is formed due to the overlap of the 1s orbital, as each atom only has one electron to donate they are effectively sharing two electrons at the same time, it is this bonding of molecular orbitals that creates a covalent bond [5].
References
- ↑ Berg J., Tymoczko J and Stryer L.(2012) Biochemistry, 7th edition, New York: WH Freeman
- ↑ Molecular Structure & Bonding (Chemistry at MSU)
- ↑ http://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/intro3.htm
- ↑ Berg, J.M., Tymoczko, J.L., and Stryer, L., 2007. Biochemistry. 6th ed. New York: W.H. Freeman. pp. 7
- ↑ Mitch fry, M.F, et al., 2008, catch up compendium for medical sciences, Banbury, scion publishing limited