Michaelas Constant
Michealas Constant (Km ) is the affinity between an enzyme and a substrate. It is the halfway point of Vmax , or can be calculated by Vmax/2.
Km is inversely proportional to the affinity between an enzyme and a substrate. The higher the Km, the lower the affinity. [1]
Finding Km
Finding Km on a Michealas-Menten graph
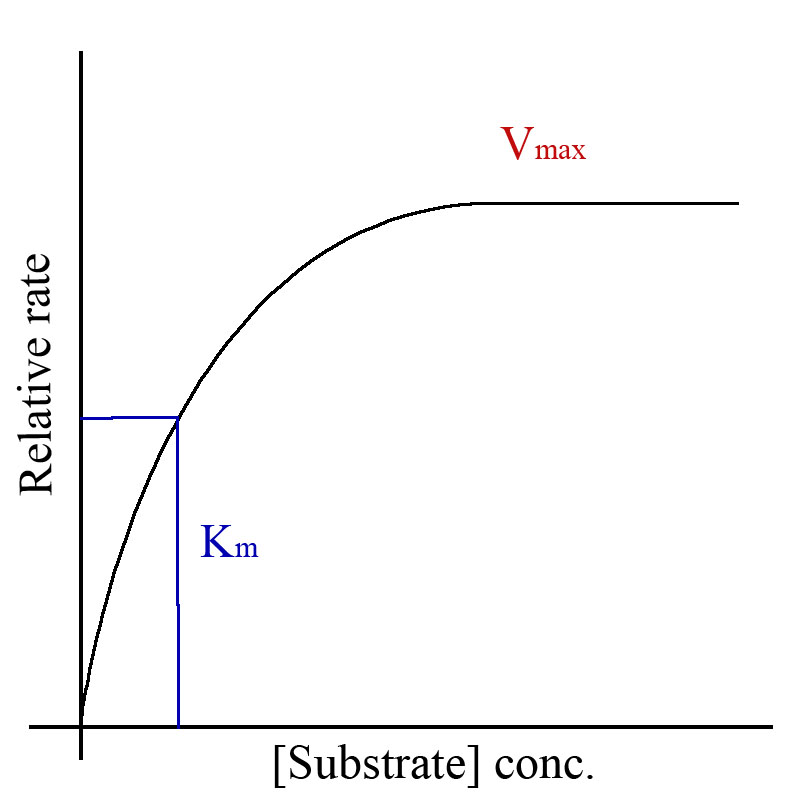
Changes in Km due to enzyme inhibition
Km may change due to inhibition of enzymes. Different kinds of inhibition leads to different changes in Km.
Competative Inhibition
During competative inhibition, the inhibitor binds to the active site of the enzyme, as it has a very similar structure to the substrate, and prevents enzyme-substrate complexes from forming. Km is increased as the inhibitors lower the affinity of the enzyme for substrates, since the enzymes tend to have higher affinity for the inhibitors.
Non-competative Inhibition
During non-competative inhibition, the inhibitor binds to an allosteric site of the enzyme, changing the same of its active site and making the active site uncompatible to substrates. Km does not change because the inhibitors do not bind to the active site.
Uncompetative Inhibition
During uncompetative inhibition, the inhibitor binds to the enzyme-substrate complex. To maintain the equailibrium between enzyme and enzyme-subtrate complex,more substrates bind to enzymes. Lower concentrations of substrates are needed to form half the maximum concentration of enzyme-substrate complex, so Km is decreased.
Reference
- ↑ Berg J., Tymoczko J. and Stryer L. (2012) Biochemistry, 7th Edition, New York: W.H. Freeman.