FRAG for Windows
Introduction
FRAG for Windows is an arithmetic program which calculates all the possible
ways of selecting atoms, so that the sum of their mass numbers equals an
input fragment mass.
 At
low resolution, integer mass, NH2 and O have the same mass of
16. N2 , CO and C2H4 all have the
same mass of 28. CF and P have the same mass of 31. When interpreting
a low-resolution mass spectrum, how can the chemist be sure to think of
all the relevant possibilities?
At
low resolution, integer mass, NH2 and O have the same mass of
16. N2 , CO and C2H4 all have the
same mass of 28. CF and P have the same mass of 31. When interpreting
a low-resolution mass spectrum, how can the chemist be sure to think of
all the relevant possibilities?
-
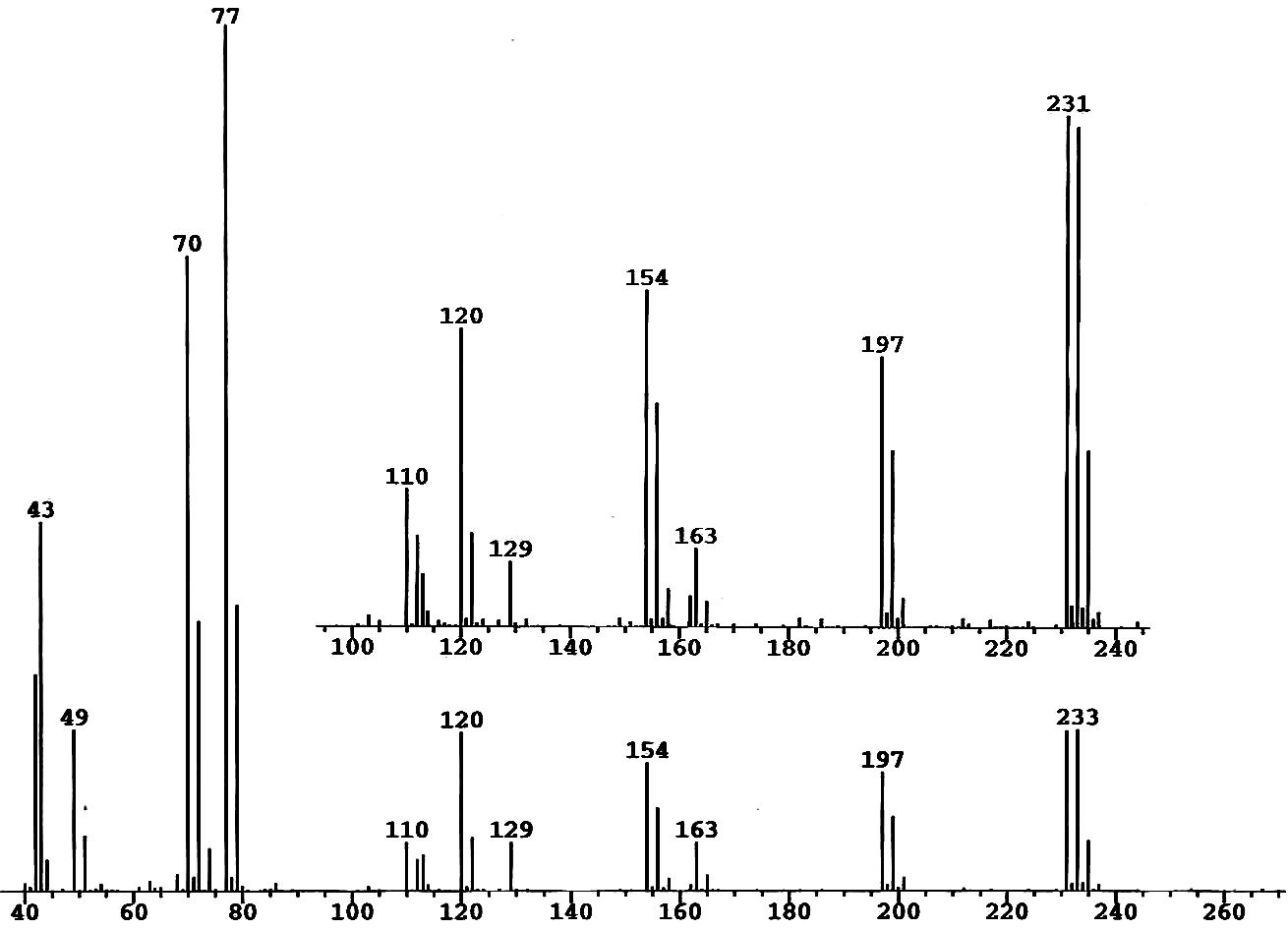
For example, consider a mass spectrum of trichlorocyanuric acid, C3N3O3Cl3,
after partial hydrolysis by adventitious water. We see a fragment
at m/z = 43 which contains no chlorine. What combination of carbon,
nitrogen, oxygen and hydrogen is this?
-
Traditionally, one used mental arithmetic to consider what combinations
of 12, 14, 16 and 1 could make up 43. It was usually possible to
arrive at one solution, but the chemist was often influenced by hypotheses
about what compounds might be present and about expected fragmentation
schemes
-
In a complex molecule, the chance of thinking of all possibilities was
not high
-
FRAG for Windows solves this problem. The user can specify that the
number of atoms of C, N, O and H are all in the range 0 - 3 inclusive
-
FRAG calculates a data structure of the possible combinations, and allows
it to be searched for the observed mass
-
m/z =43 gives four assignments. In this case, none are completely
impossible in terms of bonding, but all except one are so far removed in
possible connectivity from the molecules supposed to be present in the
sample, that they may be ruled out
-
FRAG has no valence rules built into it, so some of the assignments it
finds may be chemically impossible, even as species in a mass spectrometer
-
Users are left to use their chemical knowledge to select which of the assignments
presented by FRAG might be valid for the spectrum being interpreted
Use of FRAG with PAT for Windows
Limiting the number of impossible assignments
Using FRAG
Setup
History
Use of FRAG with PAT for Windows
When the peaks of a low-resolution mass spectrum are to be assigned to
molecular fragments, a useful first step is to use information from isotope
patterns produced by any elements in the fragment which have more than
one isotope of high abundance, e.g. chlorine, bromine, boron, zinc, or
some of the transition elements
-
This may be done with Bruce Tattershall's program PAT for Windows, which
predicts isotope patterns. Comparison of these with the observed
spectrum allows a series of peaks to be assigned to a single fragment containing
a known number of atoms of the pattern-producing element
-
Often, it is easiest to simulate patterns for the pattern-producing element
only, e.g. for Cl1 , Cl2 , Cl3 etc.
-
When an experimental pattern is seen to match one of these, one subtracts
the mass number of a particular peak of the chlorine pattern from that
of the corresponding peak of the observed pattern
-
The remaining mass corresponds to the atoms other than chlorine in the
fragment, and may then be assigned using FRAG for Windows
-
In the case given above as an example, a prominent fragment ion at m/z
= 197 showed a two-chlorine pattern
-
The pattern for Cl2 alone has its strongest peak at m/z = 70,
so that leaves m/z = 127 to assign to the remaining elements
-
FRAG for Windows shows that there is only one assignment, which is the
molecular ion of the first hydrolysis product of trichlorocyanuric acid
-
For elements such as carbon, which have only small abundances of minor
isotopes, it is best to include just 12C when using FRAG, because
the first peak of the carbon pattern, which represents fragments containing
only 12C, is much bigger than the rest, unless there is
a very large number of carbon atoms in the fragment
-
When you have assigned the first peak of a carbon pattern, you can then
put the formula of the whole fragment into PAT, and find out whether the
next peak(s) up in the observed spectrum are now also accounted for because
of the fragments which contain one (or more) atom(s) of 13C
instead of all 12C
Limiting the number of impossible assignments
Using FRAG
Setup
History
Limiting the number of impossible assignments
for organic fragment ions
-
Organic molecules, except for the smallest, contain large amounts of hydrogen
-
If one allows FRAG to calculate combinations over the full range of hydrogen
content, it will find many impossible assignments, with too many hydrogen
atoms compared with skeletal atoms
-
FRAG allows the user to set both the upper and lower limits for the numbers
of atoms of each element in the combinations to be calculated
-
A useful technique is to (a) raise the lower limit of the number of hydrogens
while the higher mass fragments are being assigned, since high-mass ions
are likely to contain substantial organic residues which retain some at
least of their hydrogen atoms; then (b) lower the lower limit and
also reduce the upper limit during the assignment of the lower mass fragments
-
This means that there will not be enough hydrogen atoms allowed to contribute
to impossible assignments for the smaller fragment ions
-
Organic chemists may also believe that substantial fragments must contain
some carbon skeleton atoms, and not be composed entirely of heteroatoms
and hydrogen
-
For this, the lower limit for the number of carbons could be set to 1,
or even to a higher number while the heavier fragments are being assigned
-
In every case, the chemist needs to use knowledge and experience before
limiting the range of solutions, otherwise some valid assignments may be
missed
Use of FRAG with PAT for Windows
Setup
History
Using FRAG
-
The program starts with the Control Panel window in focus, and with 'No.
of isotopes' highlighted
-
Enter the number of isotopes to be allowed for in the possible assignments
for observed fragments
-
E.g. if the (part of) fragments you want to assign may contain C, N or
O, enter 3
-
No. of isotopes means number of elements, if you have already dealt with
any elements which give substantial isotope patterns, using
PAT
for Windows
-
When you start to alter the table which follows, a number of lines will
become green, rather than grey, equal to the number of isotopes which you
have entered. You may alter any of the four entry boxes in each of
the lines which is green
-
If you reduce the number of isotopes after setting up all the green lines
in the table, the last line(s) of data will become grey and will not be
used by FRAG when you next Calculate possible combinations. However,
the data which you entered will still be in the greyed-out lines, and may
be used again if you later increase the number of isotopes
Entering the isotope data
-
Before clicking 'Calculate possible combinations', you must at least set
the mass number in each isotope line which is green
-
E.g. for a C N O compound, you would enter mass numbers of 12, 14 and 16
in the three green lines of the table
-
The 'Maximum no. of atoms' must be at least 1 in each green line:
if you wish to have the option of removing one of the isotopes from the
calculations easily, put it last in your list, so that it may be greyed
out by reducing 'No. of isotopes'
-
If you use a wide range of number of atoms, between 'Minimum no. of atoms'
and 'Maximum no. of atoms' inclusive, the data structure can become large,
because the number of combinations is the product of all the ranges of
the isotopes specified
-
The size of the data structure is limited only by the memory available
in the computer: if you try to exceed this, the program will terminate
with the message 'Unable to allocate storage for solutions'
-
In a modern computer, this is unlikely to happen, but setting up ranges
which are too big can result in large numbers of chemically impossible
assignments, which, by diluting the valid ones in the assignments list,
make the chemist's task more difficult and diminish the advantage of using
FRAG. See Limiting the number of impossible
assignments
-
FRAG does not use the one- or two-character 'Element symbol' you enter,
except to write it in the list of assignments, to help identify the mass
numbers of isotopes you have used. If you are sure that a group is
going to have remained intact in the range of fragment masses you are assigning,
you could invent a group symbol such as Me and use it as an 'Element symbol'
alongside the appropriate group mass number
-
When you have completed the green part of the table, click 'Calculate possible
combinations'. The number of calculated combinations is reported
to the left of the button
-
To avoid the data structure of combinations becoming out of step with alterations
to the table, the number of combinations is reset to zero every time you
make an alteration to the number of isotopes or to any of the items of
data in the green part of the table
-
Searching for assignments will then fail, until you click 'Calculate possible
combinations', to resynchronise the data structure with the setup as it
now is
Assigning a fragment mass
-
When you have clicked 'Calculate possible combinations', and therefore
have a non-zero number of combinations ready for searching, enter the first
fragment mass as 'Mass to assign' and click the 'Assign mass' button
-
Focus will switch to the Assignments for fragment mass window, where a
numbered list of assignments for the fragment mass will be displayed
-
You may return to the Control Panel window and change the 'Mass to assign'
to the next fragment to be assigned, and click the 'Assign mass' button
-
The assignments will be added to the Assignments list. When this
exceeds the length of the window, the window will scroll, so you may need
to drag the scroll bar upwards to see the start of the current list of
assignments, or to see the assignments for earlier fragments
-
The length of the list is limited only by the available computer memory,
but if it becomes too long then results are hard to relocate. A 'Clear
assignments window' button is provided on the Control Panel, to discard
all the current contents of the assignments list, and allow a fresh start
-
No facility is provided for printing the assignments list, or for copying
it to the clipboard. Usually, the user will reject the impossible
or improbable assignments, and it is easy to write down the relatively
few possible ones as formulae
Introduction
Setup
History
-
FRAG was first written as a tool for research in 1973, in HP Basic, because
the author wished to avoid some of the long periods of mental arithmetic
spent in assigning the mass spectra of new fluorocarbon-nitrogen compounds
which he was making at the time, and to be more certain of finding all
of the relevant solutions
-
In 1985 it was rewritten as a conversational program in Pascal, to take
advantage of derived data types and iterative procedures, which enabled
the data structure to be built as a binary tree, to allow fast searching
in the 8-bit Z80-based personal computers then in use
-
The Pascal code was transported to 16-bit DOS for PCs and used there for
research until the present
-
Until recently, it was thought beneficial in undergraduate teaching for
students to exercise their mental arithmetic in assigning relatively simple
mass spectra provided as exercises. However, as students have become
ever more reliant on calculators, this became slower and more difficult
for them. In 2004 we provided them with a copy of the DOS version
of FRAG, for optional use as an alternative to mental arithmetic.
It was used with great enthusiasm
-
Because of a gradual switch over from 32-bit Windows XP to 64-bit Windows
7 in Newcastle University's PC cluster teaching facilities, the ability
to run 16-bit DOS programs in teaching chemistry will soon disappear, so
the current FRAG for Windows was written in 2010. It will run in
either 32-bit or 64-bit Windows environments
-
In a modern PC, a binary tree structure is no longer necessary for fast
searching of the relatively small amount of data involved. In moving
to Fortran 90, the data structure is built as a derived-type array, with
considerable use of whole-array operations for compact and efficient code.
End-to-end searching of the array by means of a single DO loop, to find
all the assignments for a fragment mass, is almost instantaneous
-
FRAG for Windows is written in Salford ClearWin+ Fortran 90
Introduction
Using FRAG
Setup
-
This software will run in Windows95, Windows2000, Windows XP or 64-bit
Windows 7 environments
-
The screen resolution should be set to at least 800 by 600 pixels in Settings,
Control Panel, Display. At 800 by 600 pixels, the windows will just fit
on the screen. They do so with space to spare at 1024 by 768 pixels
-
The library files SALFLIBC.DLL and FTN90.DLL need to be in the windows
path or in the programs folder in which FRAGW.EXE is stored. These libraries
are commercial products, but you are permitted to take copies of them without
charge
-
The program PAT for Windows uses the same versions of the .dll files, so
if you have it in your system, the .exe file PATW.EXE may be kept conveniently
in the same programs folder as FRAGW.EXE
This software is produced in good faith with the expectation that it will
work well, but neither the author nor the University of Newcastle accepts
any liability for any failure to do so, nor for any damage to other software
or hardware which it might cause. It may not be sold to third parties
nor distributed for financial gain. Any reports on its use should
cite it as:
FRAG for Windows by B.W. Tattershall, Newcastle University, Newcastle,
England, 2010.
The author makes no commitment to remedy reported bugs or make suggested
improvements, but nevertheless would welcome comments from users.
They should be sent to:
Bruce.Tattershall@ncl.ac.uk
Introduction
Using FRAG
History
 At
low resolution, integer mass, NH2 and O have the same mass of
16. N2 , CO and C2H4 all have the
same mass of 28. CF and P have the same mass of 31. When interpreting
a low-resolution mass spectrum, how can the chemist be sure to think of
all the relevant possibilities?
At
low resolution, integer mass, NH2 and O have the same mass of
16. N2 , CO and C2H4 all have the
same mass of 28. CF and P have the same mass of 31. When interpreting
a low-resolution mass spectrum, how can the chemist be sure to think of
all the relevant possibilities?