Select the format in which molecular structures are to be shown:
- JSmol requires HTML 5.0, and can be slow
- Jmol requires Java to be installed on the client machine, but is sometimes much faster
Format currently selected: HTML5.0_JSmol
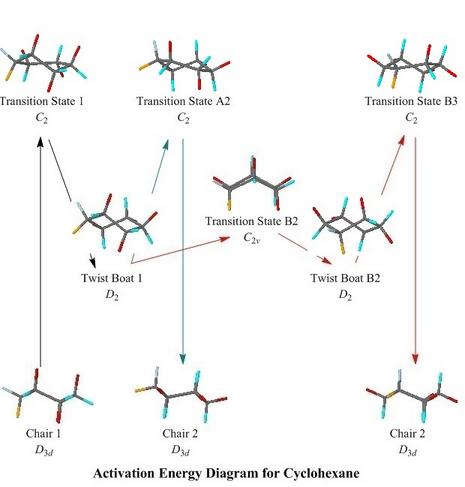
Ring Flipping of Cyclohexane
Animation using Jmol models
To view a model, click on a conformer in the schematic activation energy diagram shown
- Ignore any popup warning and click on the green Continue button which appears
Two routes are presented for transition from one chair conformation to another in which axial and
equatorial hydrogen atoms are interchanged:
- Route A (black and green arrows) goes from Chair 1 to Twist Boat 1,
one of the two enantiomeric twist boat intermediate conformers,
then directly to the other chair, Chair 2
- Route B (black and red arrows) goes from Chair 1 to Twist Boat 1,
then via non-chiral Transition State B2
to the other intermediate conformer, Twist Boat B2,
which is the enantiomer of Twist Boat 1
- Route B then continues to Chair 2
Some of the clickable conformers are specific to one route or the other. Clicking on
them will automatically change the displayed animation route if necessary. For the
others, set the desired route here:
Route currently selected: A
Mouse Control of Models
Left mouse drag rotate; Shift Left drag resize; Shift Right drag z-rotate;
Right click for menu
Notes
- The animation models are shown in two popup windows, which are reused alternately so that
you can compare one conformer or animation with another
Route A and the Chair Conformers
- Make sure that Route A is selected, then click on Chair 1
in the activation energy diagram
- The initial view for Route A models is looking down a C2 axis
which bisects C—C bonds at the front and the back of the molecule as viewed
- In the model window, you may use Shift Right drag mouse control to perform a
C2 rotation about this axis
- This C2 symmetry element is preserved through the entire reaction
path of Route A
- In either Chair form there are only two non-equivalent positions for hydrogen atoms:
they are either axial (shown as red in Chair 1) or they are equatorial (shown as
cyan). For one of the carbon atoms these are instead coloured orange and pale blue
respectively, so that you may follow a particular position on the C6 ring
during the animation
- In the model window, click the 'First frame' button to see one end of Route A, then
'Last frame' to see the other, i.e. Chair 2. The orange substituent is now equatorial
Chair Conformers of Methylcyclohexane
- In methylcyclohexane, the methyl group may be in either an axial position (e.g. the
orange substituent in Chair 1) or in an equatorial position (the pale blue substituent),
so there are two conformers of different energy
- Ring flipping allows equilibration of the chair conformers of methylcyclohexane.
The equilibrium lies strongly towards the equatorial conformer
Conformation Twist Boat 1
- View this by making sure that Route A is selected, then clicking
on Twist Boat 1 in the activation energy diagram
- Here there are three non-equivalent sets of substituents with four equivalent
positions in each
- One set is attached to carbon atoms adjacent to the C2 axis you are looking
down in the initial view of Twist Boat 1 in Route A, and the substituents are approximately
parallel to this axis.
The pair at the front of the molecule are coloured cyan, and the pair at the back are coloured red
- Another set of four substituents is attached to the same carbon atoms, but is oriented approximately
in the direction of rotation of the C2 axis. The pair at the front in the initial
view are coloured red and the pair at the back cyan
- The cyan pairs are related to the red pairs, in either of these two sets,
by a second C2 axis
which threads the centre of the six-membered ring
- The remaining set of four equivalent substituents are attached to the carbon atoms
at the extreme left and the extreme right of the initial view in Route A.
The substituents within each pair, e.g. orange and pale blue on the left of the view,
are equivalent to each other because of a third C2 axis which
passes through the leftmost and rightmost carbon atoms
- The ring is chiral because although it has these three C2 axes, it
lacks any mirror planes (or any other improper axes)
Experiments to Explore the Other Twofold Axes
- Starting from the initial view of Twist Boat 1 (refresh the model window if
necessary) drag to the right with the left mouse button, so that the model turns
about 90° around the screen y-axis, and adjust so that you are looking
exactly down the C2 axis which relates the orange substituent with
the pale blue one
- This is the 'third' C2 axis mentioned
above
- Click the First frame button, then the Last frame button. Observe that
this axis occurs only in the twist boat conformation, not in the chair conformations.
All three C2 axes in the chair conformation are through the
centres of bonds, not through carbon atoms
- Click the Starting frame button, or refresh the model page, to go back to
Twist Boat 1. Now left mouse button drag down the screen, to rotate the model
90° around the screen x-axis
- You should now be looking down the C2 axis which threads the
six-membered ring
- This axis carries all the red substituents, in each of the three sets, into
cyan substituents, and vice-versa (if you count the orange and pale blue as red and
cyan respectively)
- Try performing the C2 rotation using Shift Right mouse drag
- Again click First Frame. You should see that in the chair form, the
C2 axis is replaced by a C3 axis which carries
each red axial substituent into another one pointing in the same direction
Twist Boat Conformers of Methylcyclohexane
- The methyl group can replace hydrogen in any one of the three non-equivalent sets,
so there are three conformers with different energies
- If a chiral substituent replaces methyl, then the combination of chiral substituent
and chiral ring shape allows two diastereomers, which will have different properties,
e.g. energies. In this case then, there will be six different conformers altogether
Route B
- Transition State B2 is the untwisted boat form,
with opposite sides of the boat hull parallel to each other.
This is less stable than the
twist boats and exists only as a transition state between them,
not as an intermediate
- For Route B models the initial view is set up so that for
Transition State B2
you are looking across the straight hull of the boat, with the bow to one side and
the stern to the other
- In the activation energy diagram, click on Transition State B2 to see this
- Twist Boat 1 and Twist Boat B2 are enantiomers, with the twist in the hull of the
boat in one enantiomer being in the opposite sense to that in the other
- In the activation energy diagram, click first on Twist Boat B2 then on
Twist Boat 1, so that you may compare them between the two popup windows
The Route B view of the Chair Conformers
- In Route B, the initial views of Chair 1 or Chair B2 are along a different one
of the three C2 axes from that chosen for Route A
- This symmetry element is present only in the chair forms, and not preserved
in the twist boat conformers nor in the transition states
- In the Twist Boat 1 window, refresh it then click on First frame,
to see that now you are looking down a C2 axis. Start the
animation, and observe that this axis disappears and does not appear
until Chair B2 is reached and the animation reverses
Interconversion of the Twist Boat Forms
- The energy barrier from either of the twist boat intermediates to Transition State B2
is much less than the barrier presented by Transition State 1 or Transition State B3
respectively, so molecules which arrive in either twist boat conformation are
converted to and from the other twist boat before escaping back to a chair form
- Twist Boat 1 and Twist Boat B2 are enantiomeric for cyclohexane, as you
may verify by dragging each model with the mouse until you are looking down the
C2 axis which bisects opposite bonds (i.e. the initial view for
Route A)
- However, you may notice that the
carbon atom marked with orange and light blue substituents has moved round the ring
by one position, during the transition through Transition State B2
- Since the orange and light blue substituents were in identical environments by
symmetry in Twist Boat 1, but become in two different environments in Twist Boat B2,
the transition through the untwisted boat provides a mechanism for substituents
to appear to move to any of the three non-equivalent environments of the twist boat
shape
Experiments
- Click on Transition State B2 in the activation energy diagram, then use the mouse
to rotate the resulting model 90° about the screen x-axis, by dragging
downwards, so you are looking down into the boat
- Click Last frame and then First frame, to see the two chair end points
- Now switch to Spacefill, set the speed to 10 frames per
second, and Start the animation
- Pause the animation, and click Starting frame, to go back to Transition State B2
- Notice how close together the substituents nearest to you are: these are the
substituents at the bow and stern of the straight boat
- For bigger substituents, this steric interaction would raise the energy of this
transition state
