G-protein Coupled Receptor: Difference between revisions
No edit summary |
No edit summary |
||
| Line 13: | Line 13: | ||
= Structure = | = Structure = | ||
Along with the seven transmembrane core structure, the G Protein Coupled Receptor often have large receptor domains in the N-terminus on the extracellular side of the plasma membrane. Binding of a signal molecule to this receptor domain (or indeed the extracellular part of the transmembrane domains) cause a conformational change in the transmembrane domain and intracellular C-terminus. This triggers the action of a [[G-proteins|G-protein]] which binds guanyl nucleotides <ref>Berg, JM, Biochemistry, 6th Edition (2007), WH Freeman and Company, New York</ref>. | Along with the seven transmembrane core structure, the [[G-protein_Coupled_Receptor|G Protein Coupled Receptor]] often have large receptor domains in the N-terminus on the extracellular side of the plasma membrane. Binding of a signal molecule to this receptor domain (or indeed the extracellular part of the transmembrane domains) cause a conformational change in the transmembrane domain and intracellular C-terminus. This triggers the action of a [[G-proteins|G-protein]] which binds guanyl nucleotides <ref>Berg, JM, Biochemistry, 6th Edition (2007), WH Freeman and Company, New York</ref>. | ||
G proteins are of two types- [[Monomeric G Protein|Monomeric]] and Trimeric G proteins respectively <ref>Alberts.B Johnson.A Lewis Raff.J Roberts.K Walter.P Molecular Biology of the cell 5th edition Garland science page number 892-893</ref>.<br>Extracellular signalling molecules that bind to Enzyme-linked receptors are converted by monomeric G-proteins. <br>Example of [[ | G proteins are of two types- [[Monomeric G Protein|Monomeric]] and Trimeric G proteins respectively <ref>Alberts.B Johnson.A Lewis Raff.J Roberts.K Walter.P Molecular Biology of the cell 5th edition Garland science page number 892-893</ref>.<br>Extracellular signalling molecules that bind to Enzyme-linked receptors are converted by monomeric G-proteins. <br>Example of [[Monomeric G-protein|Monomeric G-protein]]: [[Ras|Ras]] <br>Extracellular signalling molecules that bind to G-protein linked receptors are converted by trimeric G-proteins. | ||
Trimeric G Proteins are made up of alpha beta and gamma subunits<ref>Alberts.B Johnson.A Lewis Raff.J Roberts.K Walter.P Molecular Biology of the cell 5th edition Garland science page 892</ref>. Alpha subunits have inate [[GTPase|GTPase]] activity- They bind to [[GDP|GDP]] in their resting state<ref>Alberts.B Johnson.A Lewis Raff.J Roberts.K Walter.P Molecular Biology of the cell 5th edition Garland science page 896</ref>. They are attached to the receptor at resting state in contrast to monomeric G proteins<ref>Alberts.B Johnson.A Lewis Raff.J Roberts.K Walter.P Molecular Biology of the cell 5th edition Garland science page number 892-896</ref> . The release of GDP from alpha subunit of trimeric G-protein for it to bind to [[GTP|GTP]] is driven by the G-protein linked receptor. Yet, the release of GDP of monomeric G-protein, Ras is made able by binding it to an adaptor protein, [[Grb2|Grb2]] and [[SOS|SOS]] complex (GEF[[ | Trimeric G Proteins are made up of alpha beta and gamma subunits<ref>Alberts.B Johnson.A Lewis Raff.J Roberts.K Walter.P Molecular Biology of the cell 5th edition Garland science page 892</ref>. [[alpha subunit|Alpha subunits]] have inate [[GTPase|GTPase]] activity- They bind to [[GDP|GDP]] in their resting state<ref>Alberts.B Johnson.A Lewis Raff.J Roberts.K Walter.P Molecular Biology of the cell 5th edition Garland science page 896</ref>. They are attached to the receptor at resting state in contrast to monomeric G proteins<ref>Alberts.B Johnson.A Lewis Raff.J Roberts.K Walter.P Molecular Biology of the cell 5th edition Garland science page number 892-896</ref> . The release of GDP from alpha subunit of trimeric G-protein for it to bind to [[GTP|GTP]] is driven by the G-protein linked receptor. Yet, the release of GDP of monomeric G-protein, Ras is made able by binding it to an adaptor protein, [[Grb2|Grb2]] and [[SOS|SOS]] complex (GEF[[Guanine nucleotide exchange factor|-guanine nucleotide exchange factor]]). Last but not least, GTP hydrolysis is catalysed by GTPase only in trimeric G-protein whereas GAP(GTPase Activating Protein) along with a less strong GTPase are needed to hydrolse GTP in the activated monomeric G-protein. <br> | ||
[[Image:Screen Shot 2013-10-20 at 17.30.44.png]] | [[Image:Screen Shot 2013-10-20 at 17.30.44.png]] | ||
Revision as of 11:12, 21 October 2015
The G-protein-coupled receptor (GPCR) is a seven transmembrane spanning receptor that interacts with G-protein in the process of cell signalling. It constitutes along with ion-channel-coupled receptors and enzyme-coupled receptors a major class of cell surface-receptor [1].
Classification
Over 800 G-protein-coupled receptors have been identified (more than half of them being olfactory receptors) and phylogenetic studies carried out [2]. From these studies the GPCRs can be classified in five main families:
- The rhodopsin receptor family of receptors structurally similar to rhodopsin, contains the largest number of receptors, including all the olfactory ones. Other members of this family include the adrenergic receptors, muscarinic acetylcholine receptors (mAChRs), glycoprotein-hormone receptors, serotonine receptors (except the ionotropic 5-HT3 receptor), prostaglandin receptors, thrombin receptor, etc.
- The glutamate receptor family includes the glutamate metabotropic receptors, and GABAB receptors.
- The secetrin receptor family with the receptor for the peptide hormone secretine as a prototype, it also includes the receptor for glucagon, calcitonin and parathyroid hormone.
- The adhesion receptor family characterized by the presence of motifs in the N-terminus that are likely to be related to cell adhesion.
- The Frizzled/Taste2 receptor family includes receptors important for development (frizzled branch) and the taste receptors (TAS2 branch) [3].
Structure
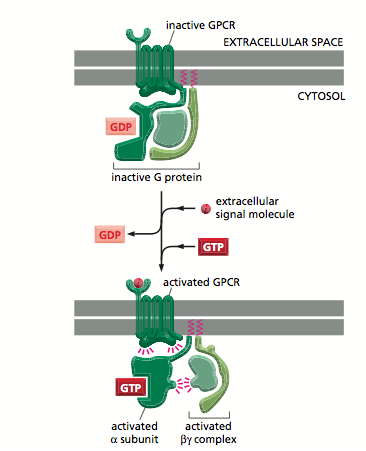
Along with the seven transmembrane core structure, the G Protein Coupled Receptor often have large receptor domains in the N-terminus on the extracellular side of the plasma membrane. Binding of a signal molecule to this receptor domain (or indeed the extracellular part of the transmembrane domains) cause a conformational change in the transmembrane domain and intracellular C-terminus. This triggers the action of a G-protein which binds guanyl nucleotides [4].
G proteins are of two types- Monomeric and Trimeric G proteins respectively [5].
Extracellular signalling molecules that bind to Enzyme-linked receptors are converted by monomeric G-proteins.
Example of Monomeric G-protein: Ras
Extracellular signalling molecules that bind to G-protein linked receptors are converted by trimeric G-proteins.
Trimeric G Proteins are made up of alpha beta and gamma subunits[6]. Alpha subunits have inate GTPase activity- They bind to GDP in their resting state[7]. They are attached to the receptor at resting state in contrast to monomeric G proteins[8] . The release of GDP from alpha subunit of trimeric G-protein for it to bind to GTP is driven by the G-protein linked receptor. Yet, the release of GDP of monomeric G-protein, Ras is made able by binding it to an adaptor protein, Grb2 and SOS complex (GEF-guanine nucleotide exchange factor). Last but not least, GTP hydrolysis is catalysed by GTPase only in trimeric G-protein whereas GAP(GTPase Activating Protein) along with a less strong GTPase are needed to hydrolse GTP in the activated monomeric G-protein.
Figure 1: showing active and inactive G-protein[9]
References
- ↑ Alberts, et al. Molecular Biology of the Cell. 5th ed. Garland Science. 2008
- ↑ Fredriksson R, Lagerström MC, Lundin LG, Schiöth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints.Mol Pharmacol. 2003 Jun;63(6):1256-72.
- ↑ Alberts.B Johnson.A Lewis Raff.J Roberts.K Walter.P Molecular Biology of the cell 5th edition Garland science page number 906 figure 15-32.
- ↑ Berg, JM, Biochemistry, 6th Edition (2007), WH Freeman and Company, New York
- ↑ Alberts.B Johnson.A Lewis Raff.J Roberts.K Walter.P Molecular Biology of the cell 5th edition Garland science page number 892-893
- ↑ Alberts.B Johnson.A Lewis Raff.J Roberts.K Walter.P Molecular Biology of the cell 5th edition Garland science page 892
- ↑ Alberts.B Johnson.A Lewis Raff.J Roberts.K Walter.P Molecular Biology of the cell 5th edition Garland science page 896
- ↑ Alberts.B Johnson.A Lewis Raff.J Roberts.K Walter.P Molecular Biology of the cell 5th edition Garland science page number 892-896
- ↑ Alberts.B Johnson.A Lewis Raff.J Roberts.K Walter.P Molecular Biology of the cell 5th edition Garland science page number 906 figure 15-32