Non-competitive inhibitor: Difference between revisions
Jump to navigation
Jump to search
Created page with "A non-competitive inhibitor binds to an enzyme in another place other than its active site. This results in a change in the enzymes structure so that it is no longer complimentry..." |
No edit summary |
||
| (8 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
A non-competitive inhibitor binds to an enzyme in another place other than its active site. This results in a change in the | [[Image:Enzyme inhibitors.gif|right|Michael-Menten Graph depicting effects of competitive and non-competitive enzyme inhibitors.]] A non-competitive inhibitor binds to an [[Enzyme|enzyme]] in another place other than its [[Active site|active site]]. This results in a change in the structure of the enzyme so that it is no longer complementary to its [[Substrate|substrate]] and therefore reduces its [[Catalytic activity|catalytic activity]]. | ||
The effects of a non-competitive inhibitor can be depicted by changes in its [[Michaelis-Menten constant|Michaelis-Menten]] graph<ref>Berg JM, Tymoczko JL, Gatto GJJ, Stayer L. Biochemistry. Eight Edition. New York: W. H. Freeman and Company; 2015</ref>: | |||
*'''[[Km|K<sub>m</sub>]] is unaltered''' as the affinity with which substrates bind to the enzyme stays the same. | |||
*'''[[Vmax|V<sub>max</sub>]] decreases''' because rate of reaction decreases due to the fact that as the reaction progresses, the concentration/amount of effective enzymes decreases. | |||
=== References === | |||
<references /> | |||
Latest revision as of 07:21, 20 October 2018
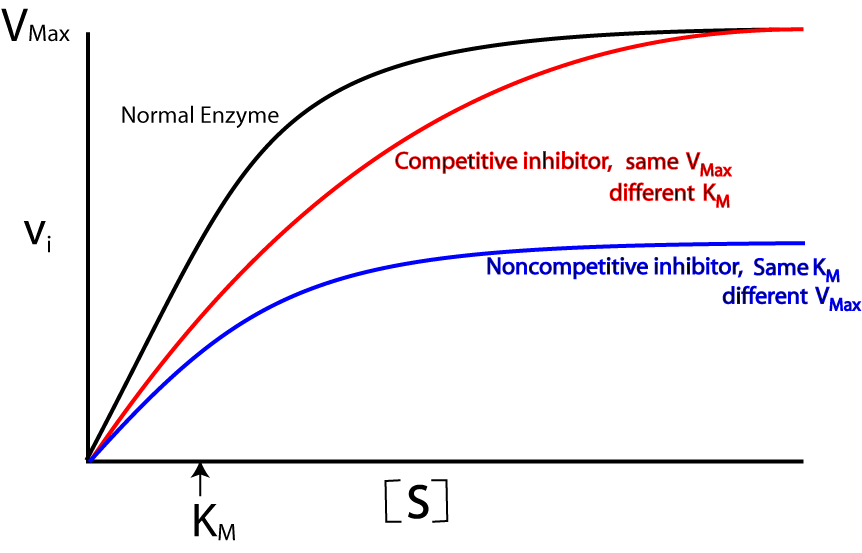
A non-competitive inhibitor binds to an enzyme in another place other than its active site. This results in a change in the structure of the enzyme so that it is no longer complementary to its substrate and therefore reduces its catalytic activity.
The effects of a non-competitive inhibitor can be depicted by changes in its Michaelis-Menten graph[1]:
- Km is unaltered as the affinity with which substrates bind to the enzyme stays the same.
- Vmax decreases because rate of reaction decreases due to the fact that as the reaction progresses, the concentration/amount of effective enzymes decreases.
References
- ↑ Berg JM, Tymoczko JL, Gatto GJJ, Stayer L. Biochemistry. Eight Edition. New York: W. H. Freeman and Company; 2015