Watson-Crick base pairs: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| (5 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
Watson-Crick base pairs is a specific complementary base pairs that base A is always paired with base T while base G is always paired with base C. | [[Image:Watson-Crick Base Pairs.PNG|left|Watson-Crick Base Pairs.PNG]]<ref>Berg, J, Stryer,L ,Tymoczko J,2012, Biochemistry, 7th edition. W. H. Freeman and Company, New York. Pg. 114, Figure 4.12.</ref>Watson-Crick base pairs is a specific complementary base pairs that base A is always paired with base T while base G is always paired with base C. These base pairs were introduced by [[James Watson|James Watson]] and [[Francis Crick|Francis Crick]]. In Watson-Crick base pairs, the number of [[Hydrogen bonds|hydrogen bonds]] formed between A and T are 2 while there are 3 formed between base G and C<ref>Berg, J, Stryer,L ,Tymoczko J,2012, Biochemistry, 7th edition. W. H. Freeman and Company, New York. Pg. 114</ref>. [[Cytosine|Cytosine]] and [[Thymine|Thymine]] are pyrimidine compounds and [[Guanine|Guanine]] and [[Adenine|Adenine]] are purine compounds. The purine compunds are larger than the pyrimidines as the 6-membered ring has an extra 5-membered ring fused to it<ref>Alberts et al. Molecular biology of the cell, fifth edition, 2007. Page 61</ref>. | ||
[[ | |||
=== References === | === References === | ||
<references /> | <references /> | ||
Latest revision as of 17:55, 22 October 2018
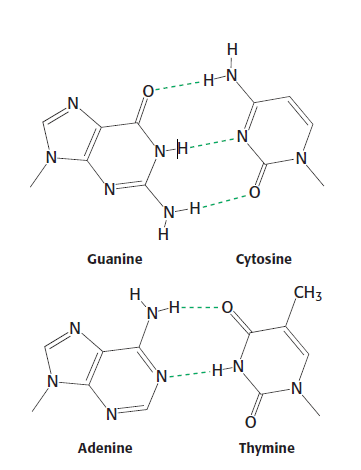
[1]Watson-Crick base pairs is a specific complementary base pairs that base A is always paired with base T while base G is always paired with base C. These base pairs were introduced by James Watson and Francis Crick. In Watson-Crick base pairs, the number of hydrogen bonds formed between A and T are 2 while there are 3 formed between base G and C[2]. Cytosine and Thymine are pyrimidine compounds and Guanine and Adenine are purine compounds. The purine compunds are larger than the pyrimidines as the 6-membered ring has an extra 5-membered ring fused to it[3].
References
- ↑ Berg, J, Stryer,L ,Tymoczko J,2012, Biochemistry, 7th edition. W. H. Freeman and Company, New York. Pg. 114, Figure 4.12.
- ↑ Berg, J, Stryer,L ,Tymoczko J,2012, Biochemistry, 7th edition. W. H. Freeman and Company, New York. Pg. 114
- ↑ Alberts et al. Molecular biology of the cell, fifth edition, 2007. Page 61