Glycosidic bond: Difference between revisions
No edit summary |
No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
Glycosidic bonds join monosaccharides or longer [[Sugar|sugar]] chains to other carbohydrates, forming disaccharides, oligosaccharides and polysaccharides. It is a type of [[Covalent bond|covalent bond]]. | Glycosidic bonds join [[Monosaccharides|monosaccharides]] or longer [[Sugar|sugar]] chains to other carbohydrates, forming [[Disaccharide|disaccharides]], [[Oligosaccharides|oligosaccharides]] and [[Polysaccharide|polysaccharides]]. It is a type of [[Covalent bond|covalent bond]]. | ||
These sugar chains of monosaccharides are able to form further glycosidic bonds with [[Alcohols|alcohols]] and [[Amines|amines]] to produce sugar acetals/glycosides and nucleosides.If the anomeric carbon of the sugar forms the bond with the oxygen atom in the hydroxyl group in the alcohol, the bond is named an O-glycosidic bond. Conversely, if the anomeric carbon of the sugar forms the bond with the nitrogen atom of an amine, the bond is then called a N-glycosidic bond | These sugar chains of [[Monosaccharides|monosaccharides]] are able to form further glycosidic bonds with [[Alcohols|alcohols]] and [[Amines|amines]] to produce sugar acetals/glycosides and nucleosides. If the anomeric carbon of the sugar forms the bond with the [[Oxygen|oxygen]] atom in the [[Hydroxyl group|hydroxyl group]] in the alcohol, the bond is named an O-glycosidic bond. Conversely, if the anomeric carbon of the sugar forms the bond with the [[Nitrogen|nitrogen]] atom of an [[Amine|amine]], the bond is then called a N-glycosidic bond <ref>'Biochemistry', Sixth Edition, (2006), Jeremy M. Berg, John L. Tymoczko and Lubert Stryer, p.309-310</ref>. | ||
<br> | <br> | ||
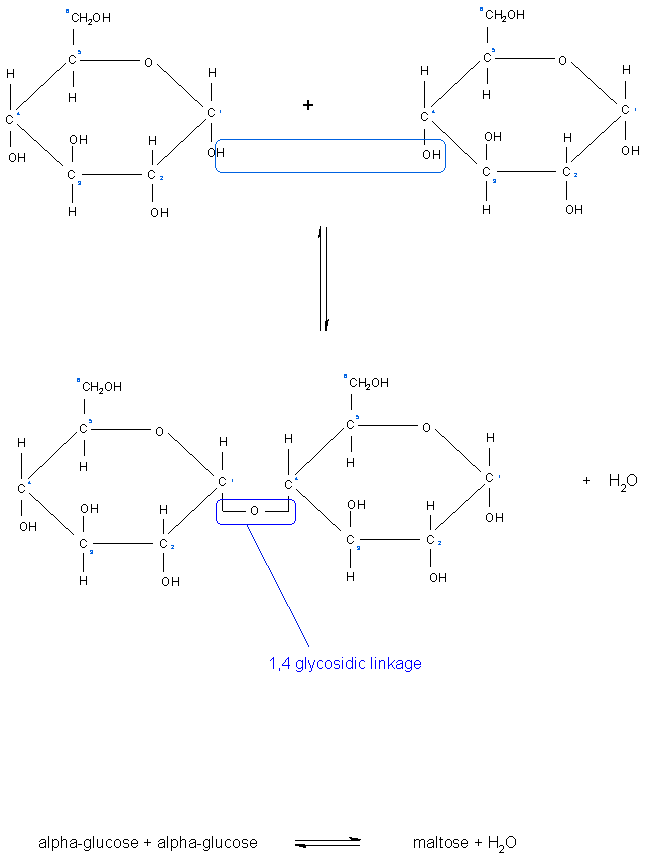
[[Image: | [[Image:Formation of glycosidic bond.gif|Formation of a glycosidic bond between two glucose sugars.]]<ref>http://www.nicksnowden.net/images/Macromolecules/maltose%20formation.GIF - 'Carbohydrates', last accessed on 10/1/11</ref><br> | ||
A Glycosidic bond is the type of linkage that occurs between sugar molecules. | |||
An [[Aldehyde|aldehyde]] or a [[Ketone|ketone]] group on the sugar can react with a [[Hydroxyl group|hydroxyl group]] on another sugar, this is what is known as a [[Glycosidic bond|glycosidic bond]]. | |||
When there are two sugar molecules linked by one [[Glycosidic bond|glycosidic link]], the resulting molecule is known as a [[Disaccharide|disaccharides]], when there are several sugar molecules linked together in this way they are known as [[Oligosaccharides|oligosaccharides]], and when there are long chains of [[Sugar|sugar]] [[Molecule|molecules]] linked in this way, they are known as [[Polysaccharide|polysaccharides]]. | |||
Glycodisic links also come in different forms and are named according to which [[Carbon|carbon]] [[Atom|atoms]] on the sugar molecule are involved in the linkage. For example, we can have 1,4 - [[Glycosidic bond|glycosidic bond]]. which involves carbon atom number 1 on one sugar and carbon atom number 4 on another sugar. These types of bonds form straight chains. There are also 1,6 - glycosidic bonds where carbon atom number 1 on one sugar is linked to carbon atom number 6 on another, this forms branch points in the molecule for example in the structure of [[Glycogen|glycogen]] and [[Starch|starch]]. The combination of 1,4 - glycosidic bonds and 1, 6 - glycosidic bonds in a polysaccharide makes it unique and chemically identifiable by specific [[Enzyme|enzymes]] and [[Receptor|receptors]] <ref>Page 113, Molecular Biology of the Cell fifth edition, Alberts et al., 2008, Garland Science, New York.</ref><br> | |||
=== References === | === References === | ||
<references /> | <references /> | ||
Latest revision as of 17:39, 10 January 2011
Glycosidic bonds join monosaccharides or longer sugar chains to other carbohydrates, forming disaccharides, oligosaccharides and polysaccharides. It is a type of covalent bond.
These sugar chains of monosaccharides are able to form further glycosidic bonds with alcohols and amines to produce sugar acetals/glycosides and nucleosides. If the anomeric carbon of the sugar forms the bond with the oxygen atom in the hydroxyl group in the alcohol, the bond is named an O-glycosidic bond. Conversely, if the anomeric carbon of the sugar forms the bond with the nitrogen atom of an amine, the bond is then called a N-glycosidic bond [1].
A Glycosidic bond is the type of linkage that occurs between sugar molecules.
An aldehyde or a ketone group on the sugar can react with a hydroxyl group on another sugar, this is what is known as a glycosidic bond.
When there are two sugar molecules linked by one glycosidic link, the resulting molecule is known as a disaccharides, when there are several sugar molecules linked together in this way they are known as oligosaccharides, and when there are long chains of sugar molecules linked in this way, they are known as polysaccharides.
Glycodisic links also come in different forms and are named according to which carbon atoms on the sugar molecule are involved in the linkage. For example, we can have 1,4 - glycosidic bond. which involves carbon atom number 1 on one sugar and carbon atom number 4 on another sugar. These types of bonds form straight chains. There are also 1,6 - glycosidic bonds where carbon atom number 1 on one sugar is linked to carbon atom number 6 on another, this forms branch points in the molecule for example in the structure of glycogen and starch. The combination of 1,4 - glycosidic bonds and 1, 6 - glycosidic bonds in a polysaccharide makes it unique and chemically identifiable by specific enzymes and receptors [3]
References
- ↑ 'Biochemistry', Sixth Edition, (2006), Jeremy M. Berg, John L. Tymoczko and Lubert Stryer, p.309-310
- ↑ http://www.nicksnowden.net/images/Macromolecules/maltose%20formation.GIF - 'Carbohydrates', last accessed on 10/1/11
- ↑ Page 113, Molecular Biology of the Cell fifth edition, Alberts et al., 2008, Garland Science, New York.