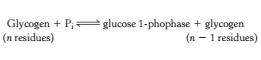Glycogenolysis: Difference between revisions
mNo edit summary |
mNo edit summary |
||
| Line 7: | Line 7: | ||
==== 1. Phosphorolysis/Shoterning of chains ==== | ==== 1. Phosphorolysis/Shoterning of chains ==== | ||
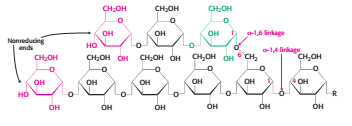
Glycogen is a branched polymer of glucose units. Glucose units in chains are linked by α-1,4-glycosidic bonds. Approximately every 10 residues of glucose, there will be a branch point created by α-1,6-glycosidic bonds. The key enzyme for glycongenolysis – glycogen phosphorylase, will cleave the terminal glucose residues which is at the non reducing end of the glycogen (i.e. cleaves α-1,4-glycosidic bonds) until four glucosyl units remain on each chain before a branch point. The non reducing end is the end of glycogen molecule with a free 4-OH group (refer to Figure 1). <br> | Glycogen is a branched polymer of glucose units. Glucose units in chains are linked by [[1,4_glycosidic_bonds|α-1,4-glycosidic bonds]]. Approximately every 10 residues of glucose, there will be a branch point created by [[1-6_glycosidic_bond|α-1,6-glycosidic bonds]]. The key enzyme for glycongenolysis – glycogen phosphorylase, will cleave the terminal glucose residues which is at the non reducing end of the glycogen (i.e. cleaves α-1,4-glycosidic bonds) until four glucosyl units remain on each chain before a branch point. The non reducing end is the end of glycogen molecule with a free 4-OH group (refer to Figure 1). <br> | ||
<br> | <br> | ||
| Line 13: | Line 13: | ||
'''[[Image:Glycogen structure.png]]'''<br> | '''[[Image:Glycogen structure.png]]'''<br> | ||
''' Figure 1 Glycogen structure <ref name="[3]">Ferrier, D.R. (2011) Lippincott’s Illustrated Reviews: Biochemistry. Sixth Edition. Philadelphia: Lippincott Williams &amp;amp;amp; Wilkins.</ref>''' | ''' Figure 1 Glycogen structure <ref name="[3]">Ferrier, D.R. (2011) Lippincott’s Illustrated Reviews: Biochemistry. Sixth Edition. Philadelphia: Lippincott Williams &amp;amp;amp;amp; Wilkins.</ref>''' | ||
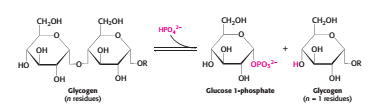
Orthophosphate (P<sub>i</sub>) – an inorganic phosphate, cleaves the the glycosidic bond between C1 of the terminal residue and C4 of the adjacent one by phosphorolysis to yield glucose 1-phosphate. The α configuration at C1 is retained even after the glycogen phosphorylase cleaves the bond between the C1 carbon atom and the glycosidic oxygen atom. (refer to Figure 2)''' ''' | Orthophosphate (P<sub>i</sub>) – an inorganic phosphate, cleaves the the glycosidic bond between C1 of the terminal residue and C4 of the adjacent one by phosphorolysis to yield glucose 1-phosphate. The α configuration at C1 is retained even after the glycogen phosphorylase cleaves the bond between the C1 carbon atom and the glycosidic oxygen atom. (refer to Figure 2)''' ''' | ||
| Line 19: | Line 19: | ||
''' '''[[Image:Equation-Glycogen(n residues) to n-1 residues.png]] | ''' '''[[Image:Equation-Glycogen(n residues) to n-1 residues.png]] | ||
'''Figure 2 Equation for phosphorolysis <ref name="[3]">Ferrier, D.R. (2011) Lippincott’s Illustrated Reviews: Biochemistry. Sixth Edition. Philadelphia: Lippincott Williams &amp;amp;amp; Wilkins.</ref>''' | '''Figure 2 Equation for phosphorolysis <ref name="[3]">Ferrier, D.R. (2011) Lippincott’s Illustrated Reviews: Biochemistry. Sixth Edition. Philadelphia: Lippincott Williams &amp;amp;amp;amp; Wilkins.</ref>''' | ||
[[Image:Glycogen.png]] | [[Image:Glycogen.png]] | ||
'''Figure 3 Phosphorolysis by an orthophosphate (inorganic phosphate) <ref name="[3]">Ferrier, D.R. (2011) Lippincott’s Illustrated Reviews: Biochemistry. Sixth Edition. Philadelphia: Lippincott Williams &amp;amp;amp; Wilkins.</ref>''' | '''Figure 3 Phosphorolysis by an orthophosphate (inorganic phosphate) <ref name="[3]">Ferrier, D.R. (2011) Lippincott’s Illustrated Reviews: Biochemistry. Sixth Edition. Philadelphia: Lippincott Williams &amp;amp;amp;amp; Wilkins.</ref>''' | ||
<br> | <br> | ||
| Line 33: | Line 33: | ||
[[Image:Glycogen remodelling.png]] | [[Image:Glycogen remodelling.png]] | ||
'''Figure 4 Glycogen remodelling (Step 1 and step 2) <ref name="[3]">Ferrier, D.R. (2011) Lippincott’s Illustrated Reviews: Biochemistry. Sixth Edition. Philadelphia: Lippincott Williams &amp;amp;amp; Wilkins.</ref>'''<br> <br> | '''Figure 4 Glycogen remodelling (Step 1 and step 2) <ref name="[3]">Ferrier, D.R. (2011) Lippincott’s Illustrated Reviews: Biochemistry. Sixth Edition. Philadelphia: Lippincott Williams &amp;amp;amp;amp; Wilkins.</ref>'''<br> <br> | ||
==== 3. Recovery'''<br>''' ==== | ==== 3. Recovery'''<br>''' ==== | ||
| Line 49: | Line 49: | ||
== Effect of hormone on glycogenolysis<br> == | == Effect of hormone on glycogenolysis<br> == | ||
Metablic effects of glucagon on glycogen breakdown – Primary target of glucagon is liver as glucagon receptors are not found on skeletal muscle. <ref name="[3]">Ferrier, D.R. (2011) Lippincott’s Illustrated Reviews: Biochemistry. Sixth Edition. Philadelphia: Lippincott Williams &amp;amp;amp; Wilkins.</ref> In fasted state, glucagon will be produced in order to increase the plasma concentration of glucose. Under the influence of glucagon, enzymes that break down glycogen become more active but the enzymes for glycogen synthesis (glycogenesis) will become less active or inhibited. | Metablic effects of glucagon on glycogen breakdown – Primary target of glucagon is liver as glucagon receptors are not found on skeletal muscle. <ref name="[3]">Ferrier, D.R. (2011) Lippincott’s Illustrated Reviews: Biochemistry. Sixth Edition. Philadelphia: Lippincott Williams &amp;amp;amp;amp; Wilkins.</ref> In fasted state, glucagon will be produced in order to increase the plasma concentration of glucose. Under the influence of glucagon, enzymes that break down glycogen become more active but the enzymes for glycogen synthesis (glycogenesis) will become less active or inhibited. | ||
Stimulation of glycogen breakdown by epinephrine/adrenaline – An epinephrine molecule binds to a β-adrenergic receptor on the plasma membrane of a liver or muscle cell. The G protein neighbouring the receptor is activated and it in turn activate stimulate the adenylyl cyclase. Adenylyl cyclase will then generate cAMP from ATP. The increase of cAMP in the cytosol will activates the protein kinase A (PKA). PKA then increases the phosphorylation of the enzyme phosphorylase kinase. Glycogen phosphorylase will then be converted from phosphorylase b, the less active form, to phosphorylase a, the more active form. The rate of glycogen breakdown will increase significantly. <ref name="[4]">Hardin, J., Bertoni, G. and Kleinsmith, L.J. (2012) Becker’s World of the Cell. Eighth Edition. San Francisco: Pearson Benjamin Cummings.</ref> | Stimulation of glycogen breakdown by epinephrine/adrenaline – An epinephrine molecule binds to a β-adrenergic receptor on the plasma membrane of a liver or muscle cell. The G protein neighbouring the receptor is activated and it in turn activate stimulate the adenylyl cyclase. Adenylyl cyclase will then generate cAMP from ATP. The increase of cAMP in the cytosol will activates the protein kinase A (PKA). PKA then increases the phosphorylation of the enzyme phosphorylase kinase. Glycogen phosphorylase will then be converted from phosphorylase b, the less active form, to phosphorylase a, the more active form. The rate of glycogen breakdown will increase significantly. <ref name="[4]">Hardin, J., Bertoni, G. and Kleinsmith, L.J. (2012) Becker’s World of the Cell. Eighth Edition. San Francisco: Pearson Benjamin Cummings.</ref> | ||
Revision as of 13:32, 18 October 2015
Definition
Glycogenolysis is defined as the fasted-state metabolism of glycogen polymers being broken down to glucose in liver, kidney or muscle or to glucose-6-phosphate for use in glycolysis pathway. [1]
Steps of glycogenolysis (glycogen breakdown)
1. Phosphorolysis/Shoterning of chains
Glycogen is a branched polymer of glucose units. Glucose units in chains are linked by α-1,4-glycosidic bonds. Approximately every 10 residues of glucose, there will be a branch point created by α-1,6-glycosidic bonds. The key enzyme for glycongenolysis – glycogen phosphorylase, will cleave the terminal glucose residues which is at the non reducing end of the glycogen (i.e. cleaves α-1,4-glycosidic bonds) until four glucosyl units remain on each chain before a branch point. The non reducing end is the end of glycogen molecule with a free 4-OH group (refer to Figure 1).
Figure 1 Glycogen structure [2]
Orthophosphate (Pi) – an inorganic phosphate, cleaves the the glycosidic bond between C1 of the terminal residue and C4 of the adjacent one by phosphorolysis to yield glucose 1-phosphate. The α configuration at C1 is retained even after the glycogen phosphorylase cleaves the bond between the C1 carbon atom and the glycosidic oxygen atom. (refer to Figure 2)
Figure 2 Equation for phosphorolysis [2]
Figure 3 Phosphorolysis by an orthophosphate (inorganic phosphate) [2]
2. Debranching/Removal of branches
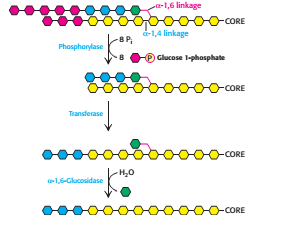
Glycogen phosphorylase can only carry out the glycogen breakdown process by itself until a limited extent before encountering an obstacle [3]. When phophorylase reaches a terminal residue four residues away from a branch point (i.e. after release of six glucose molecules), it will stop cleaving and the α-1,6 linkages are not susceptible to cleavage by phosphorylase. The branches of the glycogen molecule are removed by the debranching enzyme, a single bifunctional protein with two enzymic activities. The debranching enzyme can act as a transferase as well as an α-1,6-glucosidase to aid the continued degradation by phosphorylase. A block of three glycosyl residues from one outer branch was shifted by the transferase. The remaining single glucose molecule has a α-1,6-glycosidic bond joined to the glycogen molecule. The debranching enzyme, which act as α-1,6-glucosidase will cleave the linkage and results in the release of a free glucose molecule. The glycolytic enzyme, hexokinase will phosphorylate this free glucose molecule. Thus, the net result is a linear structure which can be continue degraded by glycogen phosphorylase.
Figure 4 Glycogen remodelling (Step 1 and step 2) [2]
3. Recovery
As in the glycolysis pathway, phosphoglucomutase is used to convert glucose 1-phosphate formed in the cleavage of glycogen into glucose 6-phosphate to enter the metabolic mainstream.
4. Release
This process will only occur in liver. In contrast with glucose, the phosphorylated glucose produce in the glycogen breakdown is not readily to be transported out of the cell. The liver contains a hydrolytic enzyme, glucose 6-phosphatase, located on the luminal side of smooth endoplasmic reticulum, which convert the glucose 6-phosphate into glucose by cleaving the phosphoryl group.
Muscle cells, lack the enzyme that can make glucose from glucose 6-phosphate. Skeletal muscle, in fasted state, will metabolise glucose 6-phosphate to either pyruvate (aerobic conditions) or lactate (anaerobic conditions). [1]
Effect of hormone on glycogenolysis
Metablic effects of glucagon on glycogen breakdown – Primary target of glucagon is liver as glucagon receptors are not found on skeletal muscle. [2] In fasted state, glucagon will be produced in order to increase the plasma concentration of glucose. Under the influence of glucagon, enzymes that break down glycogen become more active but the enzymes for glycogen synthesis (glycogenesis) will become less active or inhibited.
Stimulation of glycogen breakdown by epinephrine/adrenaline – An epinephrine molecule binds to a β-adrenergic receptor on the plasma membrane of a liver or muscle cell. The G protein neighbouring the receptor is activated and it in turn activate stimulate the adenylyl cyclase. Adenylyl cyclase will then generate cAMP from ATP. The increase of cAMP in the cytosol will activates the protein kinase A (PKA). PKA then increases the phosphorylation of the enzyme phosphorylase kinase. Glycogen phosphorylase will then be converted from phosphorylase b, the less active form, to phosphorylase a, the more active form. The rate of glycogen breakdown will increase significantly. [4]
References
- ↑ 1.0 1.1 Silverthorn, D.U. (2013) Human Physiology, An Integrated Approach. Sixth Edition. United States of America: Pearson Education.
- ↑ 2.0 2.1 2.2 2.3 2.4 Ferrier, D.R. (2011) Lippincott’s Illustrated Reviews: Biochemistry. Sixth Edition. Philadelphia: Lippincott Williams &amp;amp;amp; Wilkins.
- ↑ Berg, J.M., Tymoczko, J.L and Stryer, L. (2012) Biochemistry. Seventh Edition. New York: W.H . Freeman and Company
- ↑ Hardin, J., Bertoni, G. and Kleinsmith, L.J. (2012) Becker’s World of the Cell. Eighth Edition. San Francisco: Pearson Benjamin Cummings.