Glycine: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
Glycine has a function outside of the [[Cell|cell]]. It plays a vital role in the [[Central nervous system|central nervous system]] as is acts as a [[Neurotransmitter|neurotransmitter]] in chemical synapses<ref>Molecular biology of the cell,4th edition, 2002, Bruce Alberts, Alexander Johnson , Julian Lewis, Martin Raff , Keith Roberts and Peter Walter. Page 764</ref>. | Glycine has a function outside of the [[Cell|cell]]. It plays a vital role in the [[Central nervous system|central nervous system]] as is acts as a [[Neurotransmitter|neurotransmitter]] in chemical synapses<ref>Molecular biology of the cell,4th edition, 2002, Bruce Alberts, Alexander Johnson , Julian Lewis, Martin Raff , Keith Roberts and Peter Walter. Page 764</ref>. | ||
Glycine has two [[Hydrogen|hydrogens]] attached to the [[Alpha carbon|alpha carbon]] and is found in flexible areas of proteins due to its short side chain<ref>http://www.acnp.org/g4/gn401000008/default.htm</ref><ref>Berg, J. M., Tymoczko, J. L., and Stryer, L. (2002). Biochemistry (5th ed.). New York: W.H. Freeman</ref>. Glycine is too flexible it might distort the alpha helix.<br> | Glycine has two [[Hydrogen|hydrogens]] attached to the [[Alpha carbon|alpha carbon]] and is found in flexible areas of proteins due to its short side chain<ref>http://www.acnp.org/g4/gn401000008/default.htm</ref><ref>Berg, J. M., Tymoczko, J. L., and Stryer, L. (2002). Biochemistry (5th ed.). New York: W.H. Freeman</ref>. Glycine is too flexible it might distort the alpha helix.<ref>https://fold.it/portal/node/2000954</ref><br> | ||
=== History and etymology === | === History and etymology === | ||
Revision as of 02:42, 23 November 2018
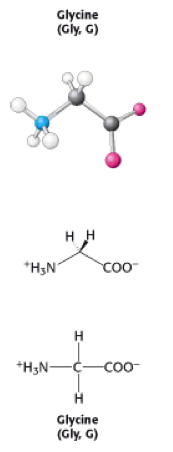
Glycine is one of the 20 amino acids. It's three letter code is Gly, and it's single letter code is G. It is the simplest amino acid, with a hydrogen atom as a side chain - this means glycine is the only amino acid which does not have a chiral carbon atom[1], so it does not form stereoisomers therefore will not have L or D configurations.
Glycine has a function outside of the cell. It plays a vital role in the central nervous system as is acts as a neurotransmitter in chemical synapses[2].
Glycine has two hydrogens attached to the alpha carbon and is found in flexible areas of proteins due to its short side chain[3][4]. Glycine is too flexible it might distort the alpha helix.[5]
History and etymology
Glycine was discovered in 1820 by Henri Braconnot when he hydrolyzed gelatin by boiling it with sulfuric acid [6]. He originally called it "sugar of gelatin"[7], but a student of Liebig showed that it contained Nitrogen, and Berzelius renamed it "glycine"[8]. The name comes from the Greek word γλυκύς "sweet tasting"[9] (which is also related to the prefixes glyco- and gluco-, as in glycoprotein and glucose). Another early name for glycine was "glycocoll"[10].
References
- ↑ Priv.-Doz. B. Kirste. (01-23-1998). Glycine. Available: http://www.chemie.fu-berlin.de/chemistry/bio/aminoacid/glycin_en.html. Last accessed 23-11-2010.
- ↑ Molecular biology of the cell,4th edition, 2002, Bruce Alberts, Alexander Johnson , Julian Lewis, Martin Raff , Keith Roberts and Peter Walter. Page 764
- ↑ http://www.acnp.org/g4/gn401000008/default.htm
- ↑ Berg, J. M., Tymoczko, J. L., and Stryer, L. (2002). Biochemistry (5th ed.). New York: W.H. Freeman
- ↑ https://fold.it/portal/node/2000954
- ↑ R.H.A. Plimmer (1912) [1908]. R.H.A. Plimmer; F.G. Hopkins, eds. The chemical composition of the proteins. Monographs on biochemistry. Part I. Analysis (2nd ed.).
- ↑ MacKenzie, Colin (1822). One Thousand Experiments in Chemistry: With Illustrations of Natural Phenomena; and Practical Observations on the Manufacturing and Chemical Processes at Present Pursued in the Successful Cultivation of the Useful Arts . Sir R. Phillips and Company.
- ↑ Nye, Mary Jo (1999). Before Big Science: The Pursuit of Modern Chemistry and Physics, 1800-1940. Harvard University Press. ISBN 9780674063822.
- ↑ "glycine". Oxford Dictionaries. Retrieved 2015-12-06.
- ↑ Ihde, Aaron J. (1970). The Development of Modern Chemistry. Courier Corporation. ISBN 9780486642352.