PH: Difference between revisions
Jump to navigation
Jump to search
Created page with ' The negative logarithm of the hydrogen ion concentration, the pH, is expressed as follows: pH = -log<sub>10</sub> [H<sup>+</sup>] The pH scale is a measure of hydrogen [[…' |
No edit summary |
||
| Line 1: | Line 1: | ||
The negative logarithm of the hydrogen ion concentration, the pH, is expressed as follows: | The negative logarithm of the hydrogen ion concentration, the pH, is expressed as follows: | ||
pH = -log<sub>10</sub> [H<sup>+</sup>] | pH = -log<sub>10</sub> [H<sup>+</sup>] | ||
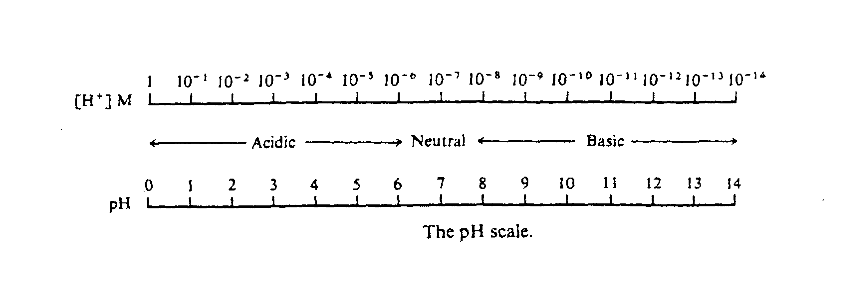
The pH scale is a measure of hydrogen [[ | The pH scale is a measure of hydrogen [[Ion|ion]] concentration that eliminates dealing with large powers of 10 and compresses a large range of concentrations onto a more convenient scale, between 1 and 14 as show in the figure below: | ||
[[Image:PH.png]]<br> | |||
Strong [[Acid|acids]] are considered to be completely dissociated into [[Ions|ions]] in dilute solutions. However, weak [[Acid|acids]] (or [[Base|bases]]) are only partially dissociated in solution, and thus an [[Equilibrium|equilibrium]] is established between the [[Ion|ions]] and the undissociated [[Molecules|molecules]]. | |||
Strong [[ | |||
Revision as of 19:21, 27 July 2010
The negative logarithm of the hydrogen ion concentration, the pH, is expressed as follows:
pH = -log10 [H+]
The pH scale is a measure of hydrogen ion concentration that eliminates dealing with large powers of 10 and compresses a large range of concentrations onto a more convenient scale, between 1 and 14 as show in the figure below:
Strong acids are considered to be completely dissociated into ions in dilute solutions. However, weak acids (or bases) are only partially dissociated in solution, and thus an equilibrium is established between the ions and the undissociated molecules.