PH: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
The negative logarithm of the hydrogen ion concentration, the pH, is expressed as follows: | The negative [[logarithm|logarithm]] of the hydrogen ion concentration, the pH, is expressed as follows: | ||
pH = -log<sub>10</sub> [H<sup>+</sup>] | pH = -log<sub>10</sub> [H<sup>+</sup>] | ||
Revision as of 19:22, 27 July 2010
The negative logarithm of the hydrogen ion concentration, the pH, is expressed as follows:
pH = -log10 [H+]
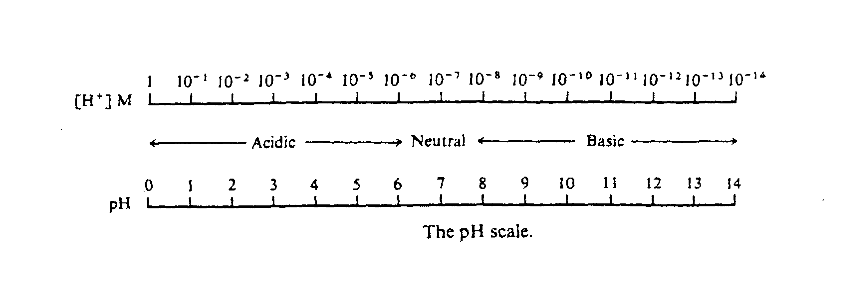
The pH scale is a measure of hydrogen ion concentration that eliminates dealing with large powers of 10 and compresses a large range of concentrations onto a more convenient scale, between 1 and 14 as show in the figure below:
Strong acids are considered to be completely dissociated into ions in dilute solutions. However, weak acids (or bases) are only partially dissociated in solution, and thus an equilibrium is established between the ions and the undissociated molecules.