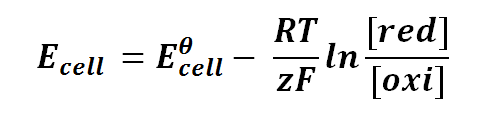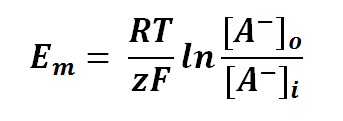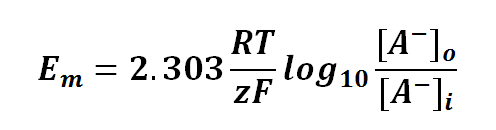Nernst Equation: Difference between revisions
No edit summary |
No edit summary |
||
| Line 41: | Line 41: | ||
<br> | <br> | ||
[[Image:Nernst equation2.png| | [[Image:Nernst equation2.png|278x98px]] | ||
<br> | <br> | ||
| Line 49: | Line 49: | ||
<br> | <br> | ||
[[Image:Nernst equation3.png| | [[Image:Nernst equation3.png|371x97px]] | ||
<br> | <br> | ||
where | where | ||
E<sub>m</sub> is the potential difference of an ion between membranes<sub></sub> | |||
R is the universal gas constant; R = 8.314471 J mol<sup>-1</sup> | |||
T is the thermodynamics temperature, in ''Kelvin''; 0 K = -273.15<sup>o</sup>C | |||
z is the number of moles of electrons transferred between membranes (defined by the valency of ion) | |||
F is the Faraday's constant; F = 96,485.3415 C mol<sup>-1</sup> | |||
[A<sup>-1</sup>] is the concentration of ion outside the membrane | |||
[A<sup>-1</sup>] is the concentration of ion inside the membrane | |||
== Application == | |||
=== Ussing Study of Frog Skin === | |||
Revision as of 21:43, 14 November 2010
Nernst Equation is an equation used to calculate the electrical potential of a chemical reaction. In its equilibrium state, the Nernst equation should be zero. It also shows the direct relation between energy or potential of a cell and its participating ions. The equation is proposed by a German chemist, Walther H. Nernst (1864-1941).
Equation
Nernst equation can be expressed as follows:
where
Ecell is the half-cell potential difference
Eθcell is the standard half-cell potential
R is the universal gas constant; R = 8.314471 J K-1 mol-1
T is the thermodynamics temperature, in Kelvin; 0 K = -273.15oC
z is the number of moles of electrons transferred between cells (defined by the valency of ions)
F is the Faraday's constant; F = 96,485.3415 C mol-1
[red] is the concentration of ion that gained electrons (reduction)
[oxi] is the concentration of ion that lost electrons (oxidation)
Membrane Potential
Nernst equation is also can be used to calculate the potential of an ion across the membrane. For potential difference of a membrane, we can manipulate the Nernst Equation as follows:
or
where
Em is the potential difference of an ion between membranes
R is the universal gas constant; R = 8.314471 J mol-1
T is the thermodynamics temperature, in Kelvin; 0 K = -273.15oC
z is the number of moles of electrons transferred between membranes (defined by the valency of ion)
F is the Faraday's constant; F = 96,485.3415 C mol-1
[A-1] is the concentration of ion outside the membrane
[A-1] is the concentration of ion inside the membrane