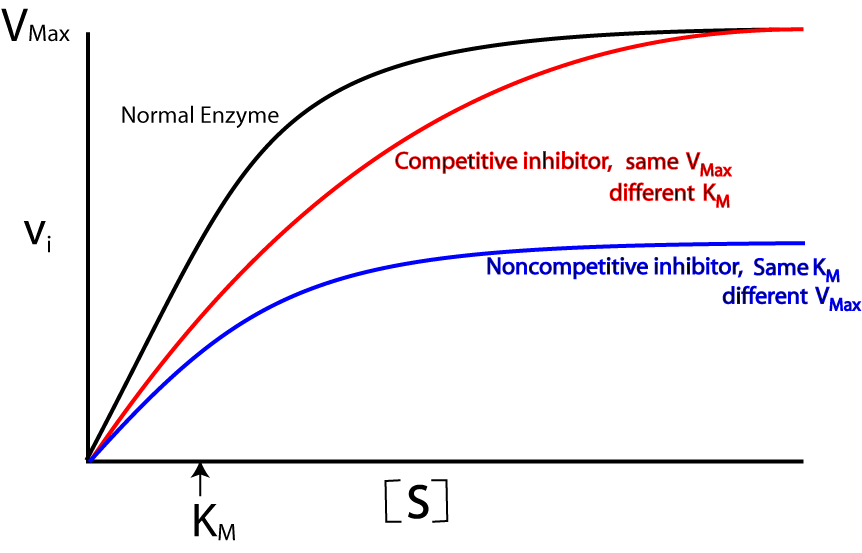
Non-competitive inhibitor
A non-competitive inhibitor binds to an enzyme in another place other than its active site. This results in a change in the enzymes structure so that it is no longer complimentry to its substrate and therefore reduces its catalytic activity.
The effects of a non-competitive inhibitor can be depicted by changes in its Michaelis-Menten graph[1]:
- Km is unaltered as the affinity with which substrates bind to the enzyme stays the same.
- Vmax decreases because rate of reaction decreases due to the fact that as the reaction progresses, the concentration/amount of effective enzymes decreases.
References
- ↑ Berg JM, Tymoczko JL, Gatto GJJ, Stayer L. Biochemistry. Eight Edition. New York: W. H. Freeman and Company; 2015