Covalent
The term 'covalent', when used within the subject of chemistry, denotes the sharing of an electron by two atoms. The repulsive force the two positive nuclei generate is countered by their mutual attraction for an electron and thus a chemical bond is formed.
Covalent bonding can be contrasted with ionic bonding which entails the electrostatic attraction of two oppositely-charged atoms due to the ‘hijacking’ of electrons by one atom from another rather than a shared pool. This can be seen in compounds such as sodium chloride (Na+Cl-) or magnesium oxide, or magnesia, (Mg2+O2-). This type of bonding confers a greater ability of the compound to dissolve in solution and so ionic compounds display a much higher propensity for dissolution than covalent species.
Covalent bonding is not limited to the sharing of one electron. In the case of a molecule of carbon dioxide (O=C=O) the carbon atom shares two electrons with each oxygen atom which also donates two electrons to the shared ‘pool’ generating noble gas configuration (a favourable state comprising 8 electrons in the external shell) for each nucleus. In the case of nitrogen gas (N![]() N) the pool is generated by each atom donating 3 electrons, attaining noble gas configuration also.
N) the pool is generated by each atom donating 3 electrons, attaining noble gas configuration also.
Using the idea of quantum electron orbitals, several specific cases of covalent bonding, notably σ-bonding (sigma bonding) and π-bonding (pi-bonding), can occur.
Sigma bonding occurs through the direct overlap of two orbitals along the same bonding plane each containing an electron of opposite spin.
Pi-bonding occurs when two p-orbitals, both perpendicular to the plane of bonding overlap; each containing electrons in opposing spins. Pi-bonding is generally weaker than sigma and only occurs once sigma bonding has already occurred.[1]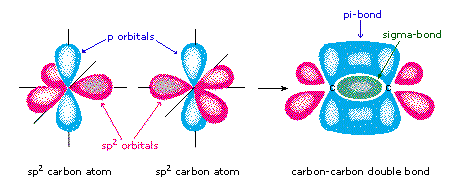
This figure shows the formation of sigma- and pi- bonds through orbital interraction.
- ↑ Molecular Structure & Bonding (Chemistry at MSU)