Beer-Lambert Law
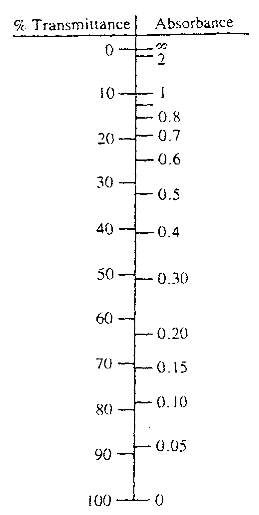
The amount of light passing through a substance is called transmittance, T or percent transmittance (%T), and is defined by the following equation:
T = I/Io, %T = I/Io x 100
Where Io is the intensity of the incident light and I is the intensity of the absorbed light[1]. The amount of light of a specified wavelength absorbed by the substance depends on the length of the light path through the substance. The negative logarithm of the transmittance, the absorbance (A), is directly proportional to the amount of light absorbed and to the length of the light path and is described by the Lambert law, which is expressed as follows:
-log T = -log I/Io = A = K1b
Here b is the length of the medium, usually, a solution in a cell and K1 is a constant.
A comparison of the scales for percent transmittance and absorbance may be used to convert percent transmittance into absorbance.
The negative logarithm of the transmittance (absorbance) is also directly proportional to the concentration of the absorbing substance c and is expressed by Beer law as follows:
-log I/Io = -log T = A = k2c
Combining the two laws as the Lambert-Beer law gives the equation:
-logI/Io = -log T = A = ε.c.l
Where ε is a constant called the extinction coefficient incorporating K1 and k2 (also called molar absorbance). The extinction coefficient is dependent on the wavelength of the light passing through the substance and on the chemical nature of the substance; l is the path length (cm) and c is the concentration of the substance[2].
Determining the Wavelength of Maximum Absorption for a Substance
To use the Lambert-Beer law to determine the concentration of a substance, light of a specified wavelength must be chosen. A spectrum of the pure substance, that is, the absorbance of the substance as a function of the wavelength of the incident light is necessary. This is most easily obtained using a recording spectrophotometer with a double beam, which is able to scan the wavelengths and thus identify the wavelength(s) of maximum absorbance.
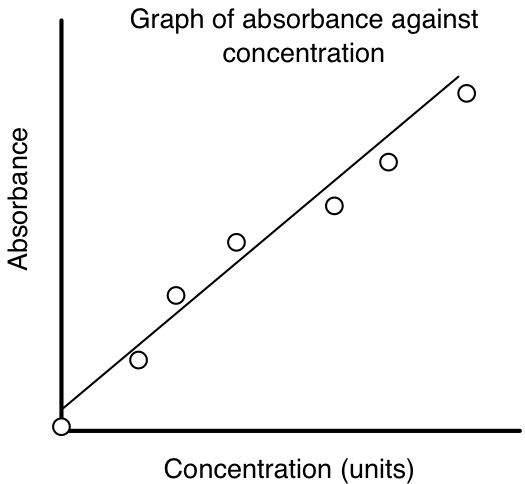
Determining the Concentration of a Substance by Spectrophotometry using a Standard Curve
Quantitation of an unknown concentration of a substance in a given biological fluid is nearly always carried out by the construction of a standard curve. In spectrophotometry, like many other analytical techniques, the instrument response (absorbance) is plotted against known concentrations of standard solutions. A plot of the absorbance versus concentration should give a linear curve whose slope is the extinction coefficient when the cell length is 1.00 cm.
The measurement of the amount of light absorbed may be either as percent transmittance (%T) or as absorbance (A). Absorbance is used more often than percent transmittance because this variable is linear with the concentration of the absorbing substance, whereas percent transmittance is exponential. The measurement of concentration is best achieved between 0.05 and 0.3 absorbance or between 90% and 50% transmittance. The errors in measuring absorbance values of 1 or 2 could be very large. The concentration of an unknown amount of a substance can be determined from a plot of the absorbance (A) versus concentration (standard curve). The readings are taken by placing the sample in a cuvette and taking the measurements, at the correct wavelength, using a spectrophotometer.
To plot a standard curve a linear curve (straight line) is drawn to best fit the scatter of data points as shown below:
When drawing a curve it is important that you:
- Use a sharp pencil to draw all lines and plot points
- If the line is supposed to be a straight line then use a ruler!
- Give the graph a title and label the axis (do not forget the units!) LIMITATIONS OF BEER LAMBERT LAW
Limitations and deviations in Beer-Lamberts law come in three forms[3]:
- Real Limitations
- Chemical Limitations
- Instrumental Limitations
Real limitations of the law arise when the concentration of the species to be analysed is high (>10mM). This is due to intermolecular forces such as charge interactions and hydrogen bonding between molecules, which can result in a shift in the absorbtion wavelength of the species.
Chemical limitations are due to intermolecular forces between the solvent and the absorbing species such as association and dissociation to create a new species with differing absorbtion characteristics.
Instrumental limitations arise from the spectrophotometers that must be used to analyse the absorbing species. Beer-Lambert law is most efficient when the radiation source being passed through it is monochromatic, however, in practice this is not possible. These limitations are overcome by using polychromatic radiation in conjunction with a filter known as a monochromator to create a monochromatic beam.
References
- ↑ Chemguide: Beer Lambert’s law. 2016. (cited 16/11/17) Available from: https://www.chemguide.co.uk/analysis/uvvisible/beerlambert.html
- ↑ Müllertz A. Perrie Y. Rades T.  Analytical Techniques in the Pharmaceutical Sciences. New York : Springer. 2016
- ↑ Pharma Xchange: Ultraviolet-Visible Spectroscopy – Limitations and Deviations of Beer-Lambert law. 2012 (cited 19/11/17) Available from: http://pharmaxchange.info/press/2012/05/ultraviolet-visible-uv-vis-spectroscopy-–-limitations-and-deviations-of-beer-lambert-law/