Free energy
Free energy is the energy in a system that is 'free' to do work, and is measured in joules.
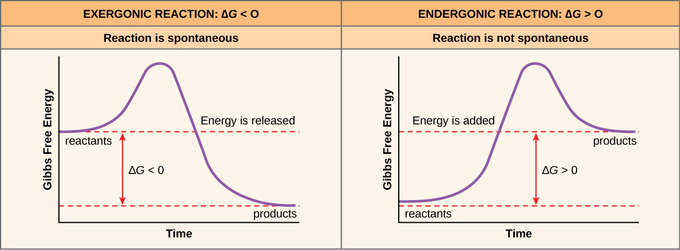
The change in free energy of a reaction is negatively proportional to the change in entropy of the universe that it causes. As a reaction only occurs spontaneously if the entropy increases, a negative change in free energy shows that a reaction can take place, and also that the overall order in the universe has increased.
Note that multi-step reactions in which one or more intermediate steps cause an increase in free energy, such as glycolysis, do not violate these rules- only the overall change in free energy must decrease for a reaction to occur spontaneously.
The convenience of using free energy over entropy is that it can be calculated using the properties of the system only and not the surroundings, making calculations and measurements simpler. It can be calculated by the following equation:
Where:
ΔG = change in free energy (joules)
ΔH = enthalpy change (joules)
T = temperature (in Kelvin)
ΔS = entropy (joules per Kelvin)
References:
- ↑ Image of graphs of Gibbs free energy taken from:fckLRfckLRBoundless (2016) Boundless Biology. Free energy. Available at: https://www.boundless.com/biology/textbooks/boundless-biology-textbook/metabolism-6/potential-kinetic-free-and-activation-energy-69/free-energy-345-11482/.