Resonance
Resonance is the delocalisation of electrons across a double bond and a single bond. In stuctures such as cyclic alkenes, e.g. benzene, the increased stability of the molecule is explained by resonance. The electrons move from the double bond where there is a large negative charge due to the higher concentration of electrons to the single bond where there is a smaller negative charge as there are fewer electrons. As a result the single bond has now became a double bond and the double bond has became a single bond. The electrons then move in reverse for the same reason and the bonds switch around again. The movement is so quick that a benzene ring, for example, could be described as having 6 'one and a half bonds' rather than locked in electrons to form 3 single bonds and 3 double bonds.
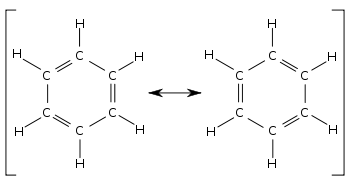 Resonance structure of benzene.[1]
Resonance structure of benzene.[1]
References
- ↑ Wikipedia. Resonance (Chemistry). Last modified 26/11/2016 [cited 5/12/16]fckLRAvailable from: https://en.wikipedia.org/wiki/Resonance_(chemistry)