Ras Guanine Nucleotide Exchange factor: Difference between revisions
Created page with "Guanine Nucleotide Exchange Factors (GEFs) are proteins that activate small monomeric GTPases, otherwise known as monomeric G-proteins. For example the Ras protein is one such GT..." |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Image:GEF.png|left|Diagram showing, very simply, the Ras-GEF interaction, allowing the release and binding of GDP and GTP respectively.]] | |||
<span style="line-height: 1.5em; font-size: 13.28px;"> | Guanine Nucleotide Exchange Factors (GEFs) are proteins that activate small monomeric [[GTPase|GTPases]], otherwise known as [[Monomeric_G-protein|monomeric G-proteins]]. For example the Ras protein is one such GTPase. The process of activation of the Ras protein begins when [[Extracellular|extracellular]][[Ras_signalling_Pathway|signaling molecules]], such as [[Growth_factor|growth factors]], bind to receptors on the phospholipid membran<span style="line-height: 1.5em; font-size: 13.28px;">e</span><ref>Alberts B., et al, 2015. Molecular Biology of the Cell. Sixth edition. New York. Garland Science. Page 157</ref><span style="font-size: 13.28px; line-height: 1.5em;">.</span> | ||
<span style="line-height: 1.5em; font-size: 13.28px;">In its inactive state, the Ras protein is strongly bound to [[GDP|guanosine diphosphate]] (GDP); Ras needs to be bound to [[GTP|guanosine triphosphate]] (GTP) in order to be activated, so that it can unleash its [[Phosphorylation|phosphorylation]] [[Phosphorylation_cascade|cascade]], activating its target protein(s). Due to this tight binding of GDP to the Ras protein, activation would be a painfully slow process without the presence of the GEF protein to allow much easier release of GDP<ref>Cherfils J. and Zeghouf M., 2013. Regulation of Small GTPases by GEFs, GAPs, and GDIs. Physiological Reviews, 93(1), page 272</ref>.</span> | |||
The GEF that allows this quicker transfer of GDP to GTP in the Ras protein was first studied in eye development of Drosophila<ref>Alberts B., et al, 2015. Molecular Biology of the Cell. Sixth edition. New York. Garland Science. Page 855</ref>. | Activation of the Ras protein is facilitated by the GEF, which binds to the inactive Ras-GDP complex and causes a conformational change. This change in the structure of the protein causes it to release GDP. The [[Active_site|active site]] of the Ras protein is now free, but because of the greater abundance of GTP in the cell compared to GDP, GTP will bind to the active site and activate the Ras protein<ref>Alberts B., et al, 2015. Molecular Biology of the Cell. Sixth edition. New York. Garland Science. Page 157</ref><span style="line-height: 1.5em; font-size: 13.28px;">.</span> | ||
The GEF that allows this quicker transfer of GDP to GTP in the Ras protein was first studied in eye development of [[Drosophila_melanogaster|Drosophila]]<ref>Alberts B., et al, 2015. Molecular Biology of the Cell. Sixth edition. New York. Garland Science. Page 855</ref>. | |||
<br> | |||
<br> | |||
<br> | |||
---- | ---- | ||
<br> | |||
<references /> | <references /> | ||
Revision as of 23:59, 16 November 2015
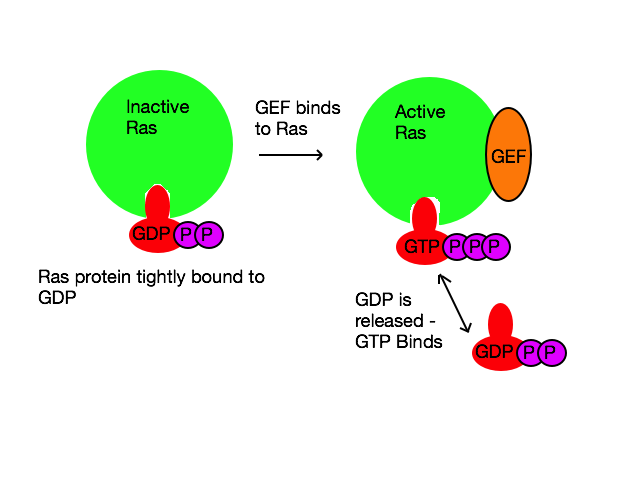
Guanine Nucleotide Exchange Factors (GEFs) are proteins that activate small monomeric GTPases, otherwise known as monomeric G-proteins. For example the Ras protein is one such GTPase. The process of activation of the Ras protein begins when extracellularsignaling molecules, such as growth factors, bind to receptors on the phospholipid membrane[1].
In its inactive state, the Ras protein is strongly bound to guanosine diphosphate (GDP); Ras needs to be bound to guanosine triphosphate (GTP) in order to be activated, so that it can unleash its phosphorylation cascade, activating its target protein(s). Due to this tight binding of GDP to the Ras protein, activation would be a painfully slow process without the presence of the GEF protein to allow much easier release of GDP[2].
Activation of the Ras protein is facilitated by the GEF, which binds to the inactive Ras-GDP complex and causes a conformational change. This change in the structure of the protein causes it to release GDP. The active site of the Ras protein is now free, but because of the greater abundance of GTP in the cell compared to GDP, GTP will bind to the active site and activate the Ras protein[3].
The GEF that allows this quicker transfer of GDP to GTP in the Ras protein was first studied in eye development of Drosophila[4].
- ↑ Alberts B., et al, 2015. Molecular Biology of the Cell. Sixth edition. New York. Garland Science. Page 157
- ↑ Cherfils J. and Zeghouf M., 2013. Regulation of Small GTPases by GEFs, GAPs, and GDIs. Physiological Reviews, 93(1), page 272
- ↑ Alberts B., et al, 2015. Molecular Biology of the Cell. Sixth edition. New York. Garland Science. Page 157
- ↑ Alberts B., et al, 2015. Molecular Biology of the Cell. Sixth edition. New York. Garland Science. Page 855