Glutamine: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| (14 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
Glutamine is one of the 20 naturally occuring [[Amino acids|amino acids]] | [[Image:Glutamine.png|right|Glutamine.png]] | ||
Glutamine is one of the 20 naturally occuring [[Amino acids|amino acids]]. It can be abbreviated to three letters: 'Gln' or one letter: 'Q' and can be encoded for by 2 different [http://teaching.ncl.ac.uk/bms/wiki/index.php/Codon codons], CAA and CAG. Glutamine is a [[Polar|polar]] [[Molecule|molecule]] meaning that it has an [[Enzyme|enzymatic]] role and can bind [[Ligand|ligands]] and other [[DNA|DNA]]. [[Polar amino acids|Polar amino acids]] are found buried in a [[Protein|protein]] and can be [[Hydrogen bonds|hydrogen-bonded]] to other [[Polar amino acids|polar amino acids]] or to the [[Polypeptide|polypeptide]] backbone<ref>Molecular biology of the cell, Alberts, 5th edition, chapter 3, page 126-129.</ref><ref>Berg, J. M., Tymoczko, J. L., and Stryer, L. (2002). Biochemistry (5th ed.). New York: W.H. Freeman.</ref>. | |||
=== References === | |||
<references /> | |||
Latest revision as of 06:09, 24 October 2018
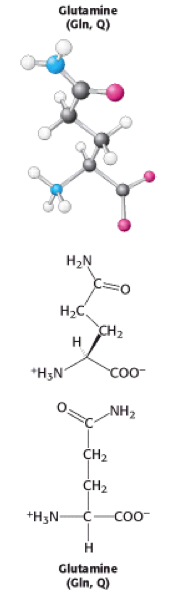
Glutamine is one of the 20 naturally occuring amino acids. It can be abbreviated to three letters: 'Gln' or one letter: 'Q' and can be encoded for by 2 different codons, CAA and CAG. Glutamine is a polar molecule meaning that it has an enzymatic role and can bind ligands and other DNA. Polar amino acids are found buried in a protein and can be hydrogen-bonded to other polar amino acids or to the polypeptide backbone[1][2].