Select the format in which molecular structures are to be shown:
- JSmol requires HTML 5.0, and can be slow
- Jmol requires Java to be installed on the client machine, but is sometimes much faster
Format currently selected: HTML5.0_JSmol
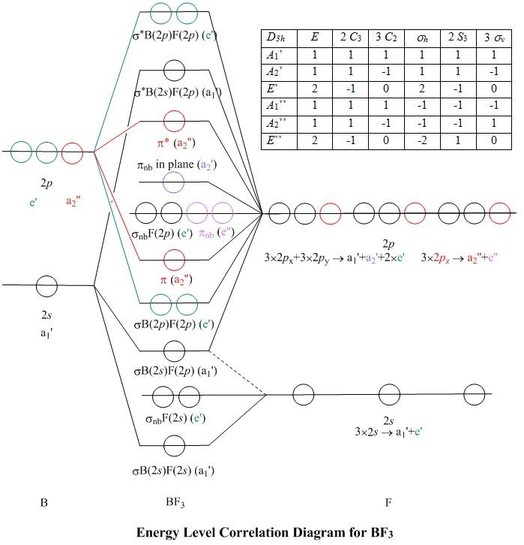
Molecular Orbitals for BF3
Jmol models of wavefunctions calculated at the RHF/3-21G* level
To view a model, click in a molecular orbital circle
in the energy level correlation diagram shown
- Ignore any popup warning and click on the green Continue button which appears
The energy level diagram may be displayed with or without the group theory symbols and
character table: the models accessed by clicking are the same.
Group theory symbols are currently onMouse Control of Models
Left mouse drag rotate; Shift Left drag resize; Shift Right drag z-rotate;
Right click for menu
Notes
- The orbitals models are shown in two popup windows, which are reused alternately so that
you can compare one orbital with another
- Contours on a two-dimensional plot correspond to surfaces in three dimensions
- The initial view of a model is with surfaces at ψ = ±0.04
- A radio button is provided to 'Switch contours on'. This shows a
two-dimensional contour plot in a plane through the boron
- This plane is xy or yz or xz, chosen so that you see some contours for each model.
This is the plane of the screen when the model first appears, though you may then turn
it around
- You may see the contour plot better if you 'Switch surfaces off' and click
'Contours coloured'. Black and white contours are provided as the default because
the coloured ones do not show up very well through the coloured surfaces
- If you 'Switch ball and stick off' you can see contours right up to the nuclei,
and where a 2s orbital contributes much you may see its inner lobe with opposite
phase
- I have named the orbitals 'sigma' or 'pi' on the energy level diagram and
in the titles on the model windows, according to common usage by inorganic chemists,
but in some cases a 'sigma' orbital is clearly σ-bonding in some part(s) of
the molecule and π-bonding in others
MO Calculation
-
These orbitals were calculated at a low ab initio level which can, however,
show bond polarisation and fully delocalised molecular orbitals
-
The method optimises orbitals filled in the ground state molecule, i.e. up to the HOMO
-
Unoccupied orbitals, i.e. the LUMO and upwards, are devised with the correct symmetry
and to be orthogonal to the filled orbitals, but at higher energies appear to become
progressively less like combinations of valence-shell Slater-type atomic orbitals
Some Other Molecules
- For a comparable group theory-based treatment of a tetratomic
but pyramidal molecule, see
PF3
- To compare orbitals for the two molecules, see the
graphical orbital-by-orbital comparison of PF3 with
BF3
- For a more extended discussion in terms of orbital symmetry,
of a molecule with fewer valence-shell MOs and no
doubly-degenerate symmetry representations, see
B2H6
