Electron transfer chain: Difference between revisions
No edit summary |
No edit summary |
||
| (4 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
The [[Electron|electron]] transfer chain is a series of protein complexes; [[NADH-Q oxioreductase|NADH-Q oxioreductase]], [[Coenzyme Q|Q-cytochrome C oxioreductase]], [[Cytochrome C oxidase|cytochrome C oxidase]] and [[ATP synthase|ATP synthase]]. In between these components are electrons carriers which will transfer the electrons from a complex to another. The protein complexes contain certain redox centres, and electrons move to carriers with increasing electron affinity along the chain: [[Image:Electron transfer chain.jpg]] | |||
[Q is coenzyme Q, also known as ubiquinone, | [Q is [[Coenzyme Q|coenzyme Q]], also known as [[Ubiquinone|ubiquinone]], carries electrons from [[NADH|NADH]] and [[FADH2|FADH<sub>2</sub>]]. It is [[Hydrophobic|hydrophobic]] and diffuses rapidly in the [[Inner mitochondrial membrane|inner mitochondrial membrane]].]<br> | ||
• ''Complex I'' – [[NADH-Q oxioreductase|NADH-Q oxioreductase]]<br> o Transfer of two high-potential electrons from [[NADH|NADH]] to [[FMN|FMN]]<br> o Electrons from FMNH<sub>2</sub> transferred to a series of Fe-S clusters<br> o Electrons from [[Fe-S clusters|Fe-S clusters]] shuttled to [[Coenzyme Q|Coenzyme Q]]<br> o Net Effect – <br> 4H<sup>+</sup> pumped out of matrix into intermembrane space<br> 2 chemical H<sup>+</sup> removed from matrix<br> | • ''Complex I'' – [[NADH-Q oxioreductase|NADH-Q oxioreductase]]<br> o Transfer of two high-potential electrons from [[NADH|NADH]] to [[FMN|FMN]]<br> o Electrons from FMNH<sub>2</sub> transferred to a series of [[Fe-S clusters|Fe-S clusters]]<br> o Electrons from [[Fe-S clusters|Fe-S clusters]] shuttled to [[Coenzyme Q|Coenzyme Q]]<br> o Net Effect – <br> 4H<sup>+</sup> pumped out of [[Mitochondrial Matrix|matrix]] into [[Mitochondrial Intermembrane Space|intermembrane space]]<br> 2 chemical H<sup>+</sup> removed from matrix<br> | ||
• ''Complex II'' – [[Succinate-Q reductase|Succinate-Q reductase]] complex<br> o [[Succinate dehydrogenase|Succinate dehydrogenase]] (in [[Citric Acid Cycle|Cirtic Acid Cycle]]) part of succinate-Q reductase complex<br> o Electrons from FADH<sub>2</sub> transferred to Fe-S clusters and then to Q<br> o ''Does not pump protons'' - therefore less ATP is formed by oxidation of FADH<sub>2</sub> than NADH<br> | • ''Complex II'' – [[Succinate-Q reductase|Succinate-Q reductase]] complex<br> o [[Succinate dehydrogenase|Succinate dehydrogenase]] (in [[Citric Acid Cycle|Cirtic Acid Cycle]]) part of succinate-Q reductase complex<br> o Electrons from FADH<sub>2</sub> transferred to Fe-S clusters and then to Q<br> o ''Does not pump protons'' - therefore less ATP is formed by [[Oxidation|oxidation]] of FADH<sub>2</sub> than NADH<br> | ||
• ''Complex III'' – [[Q-cytochrome C oxioreductase|Q-cytochrome C oxioreductase]]<br> o [[Cytochrome C|Cytochrome C]] is in all organisms with mitochondrial respiratory chains<br> o Small soluble protein containing C-type heme<br> o Carries one electron from Q-cytochrome C oxioreductase to cytochrome C oxidase<br> o Electrons transferred from QH<sub>2</sub> to oxidised cytochrome C (Cyt<sub>Cox</sub>) | • ''Complex III'' – [[Q-cytochrome C oxioreductase|Q-cytochrome C oxioreductase]]<br> o [[Cytochrome C|Cytochrome C]] is in all organisms with mitochondrial respiratory chains<br> o Small soluble protein containing [[C-type heme|C-type heme]]<br> o Carries one electron from Q-cytochrome C oxioreductase to cytochrome C oxidase<br> o Electrons transferred from QH<sub>2</sub> to oxidised cytochrome C (Cyt<sub>Cox</sub>) | ||
o Mechanism that couples electron transfer is known as the Q Cycle<br> | o Mechanism that couples electron transfer is known as the Q Cycle<br> | ||
• ''Complex IV'' – Cytochrome C Oxidase<br> o 4 electrons transferred from [[Cytochrome C|cytochrome C]] to [[Oxygen|O]]<sub>[[Oxygen|2]]</sub><br> o 4H<sup>+</sup> from matrix allow the complete [[Reduction|reduction]] of [[Oxygen|O]]<sub>[[Oxygen|2]]</sub> to [[Water|H]]<sub>[[Water|2]]</sub>[[Water|O]]<br> o 4 more H<sup>+</sup> pumped across membrane | • ''Complex IV'' – Cytochrome C Oxidase<br> o 4 electrons transferred from [[Cytochrome C|cytochrome C]] to [[Oxygen|O]]<sub>[[Oxygen|2]]</sub><br> o 4H<sup>+</sup> from matrix allow the complete [[Reduction|reduction]] of [[Oxygen|O]]<sub>[[Oxygen|2]]</sub> to [[Water|H]]<sub>[[Water|2]]</sub>[[Water|O]]<br> o 4 more H<sup>+</sup> pumped across membrane | ||
The electron motive force created by the flow of electrons down electron transport chain are used to pump H<sup>+ </sup>into the intermembrane space. This creates a concentration gradient between the inner membrane and the intermembrane space where the concentration of H in the intermembane space is higher than the concetration of H in the inner membrane space. As the inner membrane of the mitochondria is not permeable to H, this forces the H to go through the ATP synthase which will create a proton motive force to convert ADP + Pi into ATP.<br> | |||
Latest revision as of 10:00, 22 October 2018
The electron transfer chain is a series of protein complexes; NADH-Q oxioreductase, Q-cytochrome C oxioreductase, cytochrome C oxidase and ATP synthase. In between these components are electrons carriers which will transfer the electrons from a complex to another. The protein complexes contain certain redox centres, and electrons move to carriers with increasing electron affinity along the chain: 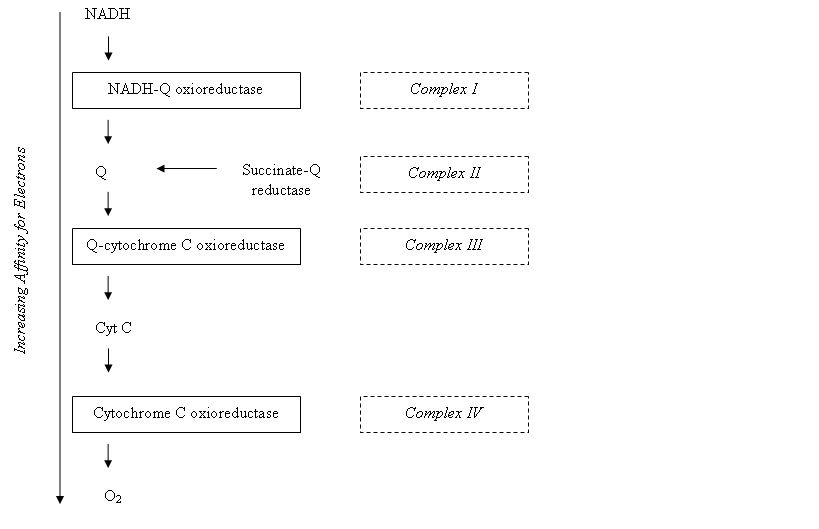
[Q is coenzyme Q, also known as ubiquinone, carries electrons from NADH and FADH2. It is hydrophobic and diffuses rapidly in the inner mitochondrial membrane.]
• Complex I – NADH-Q oxioreductase
o Transfer of two high-potential electrons from NADH to FMN
o Electrons from FMNH2 transferred to a series of Fe-S clusters
o Electrons from Fe-S clusters shuttled to Coenzyme Q
o Net Effect –
4H+ pumped out of matrix into intermembrane space
2 chemical H+ removed from matrix
• Complex II – Succinate-Q reductase complex
o Succinate dehydrogenase (in Cirtic Acid Cycle) part of succinate-Q reductase complex
o Electrons from FADH2 transferred to Fe-S clusters and then to Q
o Does not pump protons - therefore less ATP is formed by oxidation of FADH2 than NADH
• Complex III – Q-cytochrome C oxioreductase
o Cytochrome C is in all organisms with mitochondrial respiratory chains
o Small soluble protein containing C-type heme
o Carries one electron from Q-cytochrome C oxioreductase to cytochrome C oxidase
o Electrons transferred from QH2 to oxidised cytochrome C (CytCox)
o Mechanism that couples electron transfer is known as the Q Cycle
• Complex IV – Cytochrome C Oxidase
o 4 electrons transferred from cytochrome C to O2
o 4H+ from matrix allow the complete reduction of O2 to H2O
o 4 more H+ pumped across membrane
The electron motive force created by the flow of electrons down electron transport chain are used to pump H+ into the intermembrane space. This creates a concentration gradient between the inner membrane and the intermembrane space where the concentration of H in the intermembane space is higher than the concetration of H in the inner membrane space. As the inner membrane of the mitochondria is not permeable to H, this forces the H to go through the ATP synthase which will create a proton motive force to convert ADP + Pi into ATP.