Glucose: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
Glucose is a [[Monosaccharide|monosaccharide]] with the chemical formula of C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>. It is invovled in many biological processes including [[Glycolysis|glycolysis]]. [[Glycolysis|Glycolysis]] invovles the conversion of [[Glucose|glucose]], to [[Pyruvate|pyruvate]]. This process is fundamental to [[Respiration|respiration]]. Glucose can form a [[Glycosdic bond|glycosidic bond]] with another glucose to form a [[Disaccharide|disaccharide]] called [[Maltose|maltose]] through a condensation reaction. Glucose monomers can be joined by α-1,4- [[Glycosidic bond|glycosidic bond]] to form a [[Polysaccharide|polysaccharide]][[Molecule|molecule]] known as [[Starch|starch]]. <br> | Glucose is a [[Monosaccharide|monosaccharide]] with the chemical formula of C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>. It is invovled in many biological processes including [[Glycolysis|glycolysis]]. [[Glycolysis|Glycolysis]] invovles the conversion of [[Glucose|glucose]], to [[Pyruvate|pyruvate]]. This process is fundamental to [[Respiration|respiration]]. Glucose can form a [[Glycosdic bond|glycosidic bond]] with another glucose to form a [[Disaccharide|disaccharide]] called [[Maltose|maltose]] through a condensation reaction. Glucose monomers can be joined by α-1,4- [[Glycosidic bond|glycosidic bond]] to form a [[Polysaccharide|polysaccharide]][[Molecule|molecule]] known as [[Starch|starch]]. <br> | ||
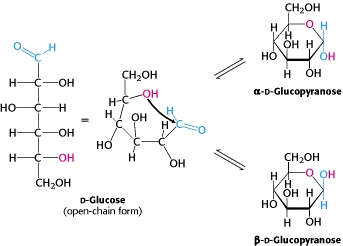
In open chain [[Glucose|glucose]] Carbon 1 (the [[Carbonyl group|carbonyl]] carbon) is not [[Chiral centre|chirally]] active. However in the ring structure it becomes assymetric, allowing it to form two ring structures: [[ | In open chain [[Glucose|glucose]] Carbon 1 (the [[Carbonyl group|carbonyl]] carbon) is not [[Chiral centre|chirally]] active. However in the ring structure it becomes assymetric, allowing it to form two ring structures: [[Α-D-glucopyranose|α-D-glucopyranose]] and [[Β-D-glucopyranose|β-D-glucopyranose]]. <ref>Berg J., Tymoczko J and Stryer L. (2012) Biochemistry, 7th edition, New York: WH Freeman. pg 333</ref><br> | ||
[[Image:D-glucose.jpg|D-glucose forming alpha and beta rings. ]] | |||
Taken from: [http://www.ncbi.nlm.nih.gov/books/NBK22547/ http://www.ncbi.nlm.nih.gov/books/NBK22547/]<ref>http://www.ncbi.nlm.nih.gov/books/NBK22547/</ref><br> | |||
The main family of transporters are known as the GLUT family with 5 known varients all with different properties and found in different tissues. | The main family of transporters are known as the GLUT family with 5 known varients all with different properties and found in different tissues. | ||
Revision as of 14:47, 30 November 2012
Glucose is a monosaccharide with the chemical formula of C6H12O6. It is invovled in many biological processes including glycolysis. Glycolysis invovles the conversion of glucose, to pyruvate. This process is fundamental to respiration. Glucose can form a glycosidic bond with another glucose to form a disaccharide called maltose through a condensation reaction. Glucose monomers can be joined by α-1,4- glycosidic bond to form a polysaccharidemolecule known as starch.
In open chain glucose Carbon 1 (the carbonyl carbon) is not chirally active. However in the ring structure it becomes assymetric, allowing it to form two ring structures: α-D-glucopyranose and β-D-glucopyranose. [1]
Taken from: http://www.ncbi.nlm.nih.gov/books/NBK22547/[2]
The main family of transporters are known as the GLUT family with 5 known varients all with different properties and found in different tissues.
References:
- ↑ Berg J., Tymoczko J and Stryer L. (2012) Biochemistry, 7th edition, New York: WH Freeman. pg 333
- ↑ http://www.ncbi.nlm.nih.gov/books/NBK22547/