Guanosine triphosphate
Guanosine triphosphate (Guanosine-5'-triphosphate to be precise or also commonly abbreviated GTP for simplicity) is a high energy nucleotide (not to be confused with nucleoside). As a result of it's structure it has selective roles in the formation of RNA strands[1], functioning

as an energy carrier molecule for protein synthesis[2], a coenzyme, a predecessor to cGMP - a secondary messenger molecule[3] or as an effector molecule. The last both of which are demonstrated by G-protein modulation[4]. This list does not exhuast it's chemical interactions but is merely a demonstration of it's capabilities.
The ribose sugar is central to the three dimensional arrangement of the covalently bonded guanine and the triphosphate, providing hydroxyl groups for condensation reactions and nucleophilic attacks[5].The guanine molecule and the triphosphate form covalent bonds at C'1 and C'5 atoms respectively. The purine is bonded as a result of a condensation reaction at it's 9'N. Since guanine is a purine base, it is classified as a purine triphosphate along with adenine triphosphate (ATP)[6]. It is formed along with ATP through inosine monophosphate modification[7]. It's structural formula (right) suggests it's chemical activity and is described further in detail below.
Misconception: GTP, A Baseline Building Block
The liver is the principal organ which synthesises nucleotides. It does so by first creating inosine monophosphate from ATP, glutamine, glycine, CO2, aspartate and formate[8]. IMP can then be modified to yield any purine nucleotide. In the case of GTP formation, IMP is first converted into XMP by IMP dehydrogenase. This allows for the action of GTP synthase which rapidly converts XMP into GMP[9]. GMP is then phosphorylated by nucleoside phosphate kinases to yield diphosphates and eventually triphosphates.
GTP is catabolised into insoluble uric acid which is present in the urine as sodium urate crystals[10]. Gout is a manifestation of the abnormal catabolism of purine triphosphates in the synovial joints. This leads to the presence of monosodium urate or calcium pyrophosphate dihydrate which causes clinical symptoms of inflammation and arthritis[11]. Severe combined immunodeficieny disease also can occur as a result of abnormal catabolism of purine triphosphates, which results in the destruction of B and T lymphocytes[12].
One Of Many RNA Base Predecesors
RNA is chemically distinct from DNA primarily as a result of the existance of a deoxyribose instead of a ribose sugar. Guanosine triphosphate is concerned with the production of the guanine base only in RNA[13]. In DNA deoxyguanosine triphosphates are used instead, as they do not possess a 2'OH group which makes them prone to nucleophilic attacks which can result in the hydrolysis of the base from the rest of the polynucleotide.
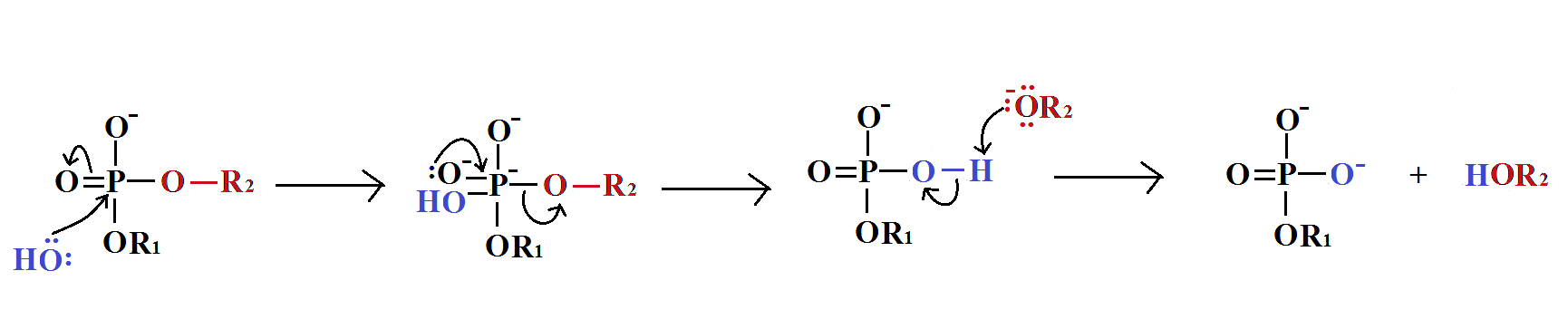
Guanosine triphosphate will result in the formation of a guanine base as a result of cleavage of two anhydride bonds, releasing two free phosphates as products. However, this reaction will (normally) only be catalysed byRNA polymerase if the opposite base is a cytosine with which the guanosine triphosphate can form hydrogen bonds. It should be noted that this occurs independantly from the action of RNA polymerase, which merely forms phosphodiester bonds between already aligned triphosphates. After catalysis, the molecule is part of a polynucleotide chain and is no longer known as GTP, but as the base guanine.
GTP; A Cousin of Universal ATP
Suprising Signal Transduction Effector
References
- ↑ J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.
- ↑ R K Murray, D A Bender, K M Botham, P J Kennelly, V W Rodwell and P A Weil. Harper's Illustrated Biochemistry. 28th Edition. Beijing, China. 2009.
- ↑ J Dow, G Lindsay and J Morrison, Biochemistry: Molecules, Cells and the Body. 1st Edition. Wokingham, England. Addison-Wesley. 1996.
- ↑ J Dow, G Lindsay and J Morrison, Biochemistry: Molecules, Cells and the Body. 1st Edition. Wokingham, England. Addison-Wesley. 1996.
- ↑ J Dow, G Lindsay and J Morrison, Biochemistry: Molecules, Cells and the Body. 1st Edition. Wokingham, England. Addison-Wesley. 1996.
- ↑ J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.
- ↑ J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.
- ↑ M W King. The Medical Biochemistry Page. September 2016. Cited: 11:31. 03.12.2016. Available from: http://themedicalbiochemistrypage.org/nucleotide-metabolism.php#purine
- ↑ M W King. The Medical Biochemistry Page. September 2016. Cited: 11:31. 03.12.2016. Available from: http://themedicalbiochemistrypage.org/nucleotide-metabolism.php#purine
- ↑ M W King. The Medical Biochemistry Page. September 2016. Cited: 11:31. 03.12.2016. Available from: http://themedicalbiochemistrypage.org/nucleotide-metabolism.php#purine
- ↑ M W King. The Medical Biochemistry Page. September 2016. Cited: 11:31. 03.12.2016. Available from: http://themedicalbiochemistrypage.org/nucleotide-metabolism.php#purine
- ↑ M W King. The Medical Biochemistry Page. September 2016. Cited: 11:31. 03.12.2016. Available from: http://themedicalbiochemistrypage.org/nucleotide-metabolism.php#purine
- ↑ J Dow, G Lindsay and J Morrison, Biochemistry: Molecules, Cells and the Body. 1st Edition. Wokingham, England. Addison-Wesley. 1996.
SaveSave