Phosphodiester bond: Difference between revisions
Created page with 'The phosphodiester bond links two pentose sugars to a phosphate groups by strong, covalent ester bonds. The formation of these bonds is a condensation reaction in which water is …' |
No edit summary |
||
| (9 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
A phosphodiester bond occurs when exactly two of the hydroxyl groups in phosphoric acid react with hydroxyl groups on other molecules to form two ester bonds. An example is found in the linking of two [[Pentose|pentose ]](5 carbon sugar) rings to a [[Phosphate group|phosphate group]] by strong, [[Covalent|covalent]] [[Ester bond|ester bonds]]. Each ester bond is formed by a [[Condensation Reaction|condensation reaction]] in which water is lost. This bond is a key structural feature of the backbone of DNA and RNA and links the 3’ carbon of one nucleotide to the 5’ carbon of another to produce the strands of [[DNA|DNA and]] [[RNA|RNA]]. | |||
= Phosphodiester Bond Formation = | = Phosphodiester Bond Formation = | ||
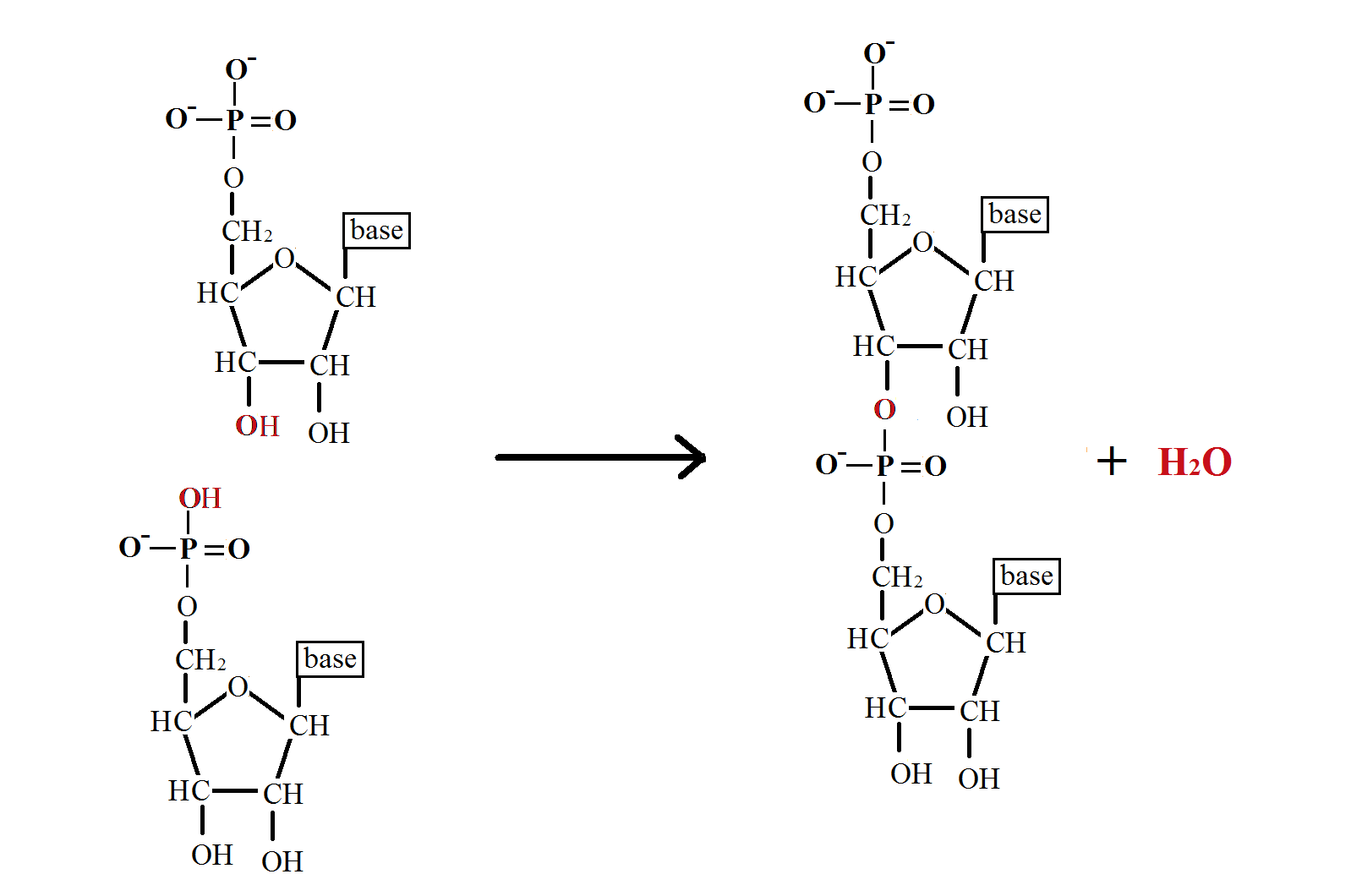
In phosphodiester formation, | In phosphodiester formation, two [[Hydroxyl groups|hydroxyl ]](OH) groups on the phosphate molecule bind to the 3’ and 5’ carbons on two independent pentose sugars. These are two condensation reactions, so two molecules of water are produced. The phosphate is then bonded to the sugars by two ester bonds, hence the nomenclature of phosphodiester bond. This reaction is catalysed by [[Ligase|ligases]], such as [[Dna ligase|DNA ligase]] during [[DNA_replication|DNA replication]]. <br> | ||
A representation of the reaction is shown in the diagram below. | |||
[[Image:Phosphodiester formation mechanism.png|774x512px|Phosphodiester formation mechanism.png]] | |||
[[Image: | <br> | ||
= <br>Phosphodiester Bond Hydrolysis = | |||
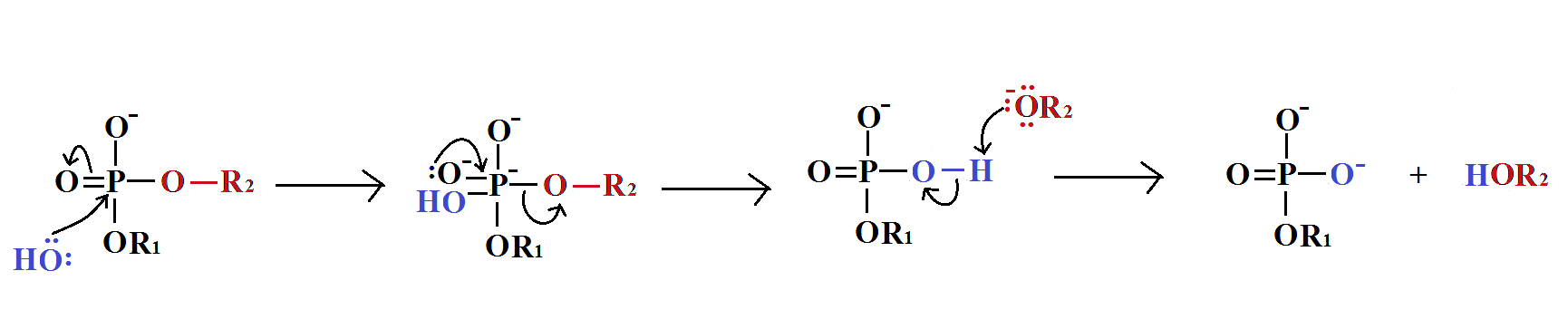
In phosphodiester [[Hydrolysis|hydrolysis]], water is dissociated into H<sup><sub>+ </sub></sup>and OH<sup>-</sup>. The OH<sup>-</sup> acts as a [[Nucleophile|nucleophile]] in the reaction. The reaction is catalysed by [[Phosphodiesterase|phosphodiesterase]].<br>The mechanism of this reaction is given below. | |||
[[Image:Phosphodiester hydrolysis mechanism.png|992x208px|Phosphodiester hydrolysis mechanism.png]]<br><br> | |||
Latest revision as of 15:35, 30 November 2012
A phosphodiester bond occurs when exactly two of the hydroxyl groups in phosphoric acid react with hydroxyl groups on other molecules to form two ester bonds. An example is found in the linking of two pentose (5 carbon sugar) rings to a phosphate group by strong, covalent ester bonds. Each ester bond is formed by a condensation reaction in which water is lost. This bond is a key structural feature of the backbone of DNA and RNA and links the 3’ carbon of one nucleotide to the 5’ carbon of another to produce the strands of DNA and RNA.
Phosphodiester Bond Formation
In phosphodiester formation, two hydroxyl (OH) groups on the phosphate molecule bind to the 3’ and 5’ carbons on two independent pentose sugars. These are two condensation reactions, so two molecules of water are produced. The phosphate is then bonded to the sugars by two ester bonds, hence the nomenclature of phosphodiester bond. This reaction is catalysed by ligases, such as DNA ligase during DNA replication.
A representation of the reaction is shown in the diagram below.
Phosphodiester Bond Hydrolysis
In phosphodiester hydrolysis, water is dissociated into H+ and OH-. The OH- acts as a nucleophile in the reaction. The reaction is catalysed by phosphodiesterase.
The mechanism of this reaction is given below.