Guanosine triphosphate: Difference between revisions
Removed some weird code on the end. Good edit! |
No edit summary |
||
| Line 1: | Line 1: | ||
Guanosine triphosphate (Guanosine-5'-triphosphate to be precise or also commonly abbreviated GTP for simplicity) is a high energy [[Nucleotide|nucleotide]] (not to be confused with [[Nucleoside|nucleoside]]). | Guanosine triphosphate (Guanosine-5'-triphosphate to be precise or also commonly abbreviated GTP for simplicity) is a high energy [[Nucleotide|nucleotide]] (not to be confused with [[Nucleoside|nucleoside]]). | ||
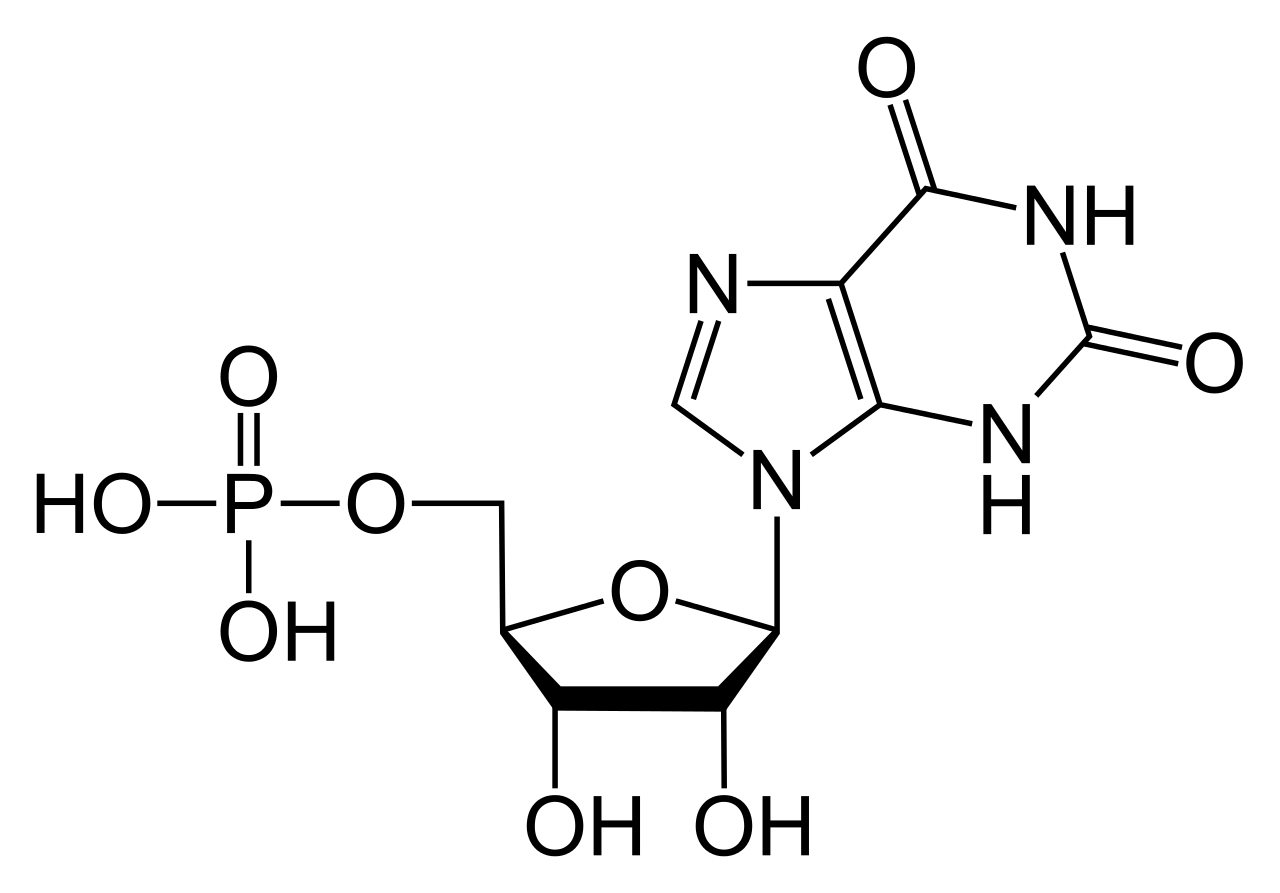
As a result of it's structure it has selective roles in the formation of [[MRNA|RNA]] strands<ref>J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.</ref>, functioning | [[Image:GTP chemical structure.png|frame|right|300x200px]]<span style="font-size: 13.28px;" /> | ||
As a result of it's structure it has selective roles in the formation of [[MRNA|RNA]] strands<ref>J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.</ref>, functioning as an [[Energy carrier|energy carrier]] molecule for protein synthesis<ref>R K Murray, D A Bender, K M Botham, P J Kennelly, V W Rodwell and P A Weil. Harper's Illustrated Biochemistry. 28th Edition. Beijing, China. 2009.</ref>, a [[Coenzyme|coenzyme]], a predecessor to cGMP - a [[Secondary messenger|secondary messenger]] molecule<ref>J Dow, G Lindsay and J Morrison, Biochemistry: Molecules, Cells and the Body. 1st Edition. Wokingham, England. Addison-Wesley. 1996.</ref> or as an [[Effector|effector]] molecule. The last two are commonly demonstrated by [[G-protein|G-protein]] modulation<ref>J Dow, G Lindsay and J Morrison, Biochemistry: Molecules, Cells and the Body. 1st Edition. Wokingham, England. Addison-Wesley. 1996.</ref>. It is important to note that this list does not exhuast it's chemical interactions but is merely a demonstration of it's various capabilities. | |||
In GTP the [[Ribose|ribose]] sugar is central to the three dimensional arrangement of the covalently bonded [[Guanine|guanine]] and [[T|t]][[Triphosphate|riphosphate]] molecules. This monosaccharide provide [[Hydroxyl group|hydroxyl]] groups for [[Condensation Reaction|condensation reactions]] and [[Nucleophilic attack|nucleophilic attacks]]<ref>J Dow, G Lindsay and J Morrison, Biochemistry: Molecules, Cells and the Body. 1st Edition. Wokingham, England. Addison-Wesley. 1996.</ref>.The guanine molecule and the triphosphate form covalent bonds at [[C|C'1]] and [[C|C'5]] atoms respectively. The purine is bonded as a result of a condensation reaction at it's [[Nitrogen|9'N]]. Since guanine is a [[Purine|purine]] base, it is classified as a purine triphosphate along with [[Adenine|a]][[Adenine triphosphate|denine triphosphate (ATP)]]<ref>J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.</ref>. It is formed along with [[ATP|ATP]] through [[Inosine monophosphate|inosine monophosphate]] modification<ref>J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.</ref>. It's [[Structural formula|structural formula ]](right) suggests it's chemical activity and is described further in detail below. <br> | In GTP the [[Ribose|ribose]] sugar is central to the three dimensional arrangement of the covalently bonded [[Guanine|guanine]] and [[T|t]][[Triphosphate|riphosphate]] molecules. This monosaccharide provide [[Hydroxyl group|hydroxyl]] groups for [[Condensation Reaction|condensation reactions]] and [[Nucleophilic attack|nucleophilic attacks]]<ref>J Dow, G Lindsay and J Morrison, Biochemistry: Molecules, Cells and the Body. 1st Edition. Wokingham, England. Addison-Wesley. 1996.</ref>.The guanine molecule and the triphosphate form covalent bonds at [[C|C'1]] and [[C|C'5]] atoms respectively. The purine is bonded as a result of a condensation reaction at it's [[Nitrogen|9'N]]. Since guanine is a [[Purine|purine]] base, it is classified as a purine triphosphate along with [[Adenine|a]][[Adenine triphosphate|denine triphosphate (ATP)]]<ref>J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.</ref>. It is formed along with [[ATP|ATP]] through [[Inosine monophosphate|inosine monophosphate]] modification<ref>J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.</ref>. It's [[Structural formula|structural formula ]](right) suggests it's chemical activity and is described further in detail below. <br> | ||
=== Misconception: GTP, A Baseline Building Block === | === Misconception: GTP, A Baseline Building Block === | ||
The [[Liver|liver]] is the principal organ which synthesises nucleotides. It does so by first creating [[Inosine|inosine monophosphate]] from [[ATP|ATP]], [[Glutamine|glutamine]], [[Glycine|glycine]], [[CO2|CO2]], [[Aspartate|aspartate]] and [[Formate|formate]]<ref>M W King. The Medical Biochemistry Page. September 2016. Cited: 11:31. 03.12.2016. Available from: http://themedicalbiochemistrypage.org/nucleotide-metabolism.php#purine</ref>. IMP can then be modified to yield any purine nucleotide. In the case of GTP formation, IMP is first converted into [[XMP|XMP]] by IMP [[Dehydrogenase|dehydrogenase]]. This allows for the action of GTP synthase which rapidly converts XMP into GMP<ref>M W King. The Medical Biochemistry Page. September 2016. Cited: 11:31. 03.12.2016. Available from: http://themedicalbiochemistrypage.org/nucleotide-metabolism.php#purine</ref>. GMP is then phosphorylated by nucleoside phosphate kinases to yield diphosphates and eventually triphosphates. | [[Image:Xanthosine_monophosphate.svg.png|frame|left|200x150px|The structural formula of XMP. Note the differences between it and GTP's nitrogenous base.]]The [[Liver|liver]] is the principal organ which synthesises nucleotides. It does so by first creating [[Inosine|inosine monophosphate]] from [[ATP|ATP]], [[Glutamine|glutamine]], [[Glycine|glycine]], [[CO2|CO2]], [[Aspartate|aspartate]] and [[Formate|formate]]<ref>M W King. The Medical Biochemistry Page. September 2016. Cited: 11:31. 03.12.2016. Available from: http://themedicalbiochemistrypage.org/nucleotide-metabolism.php#purine</ref>. IMP can then be modified to yield any purine | ||
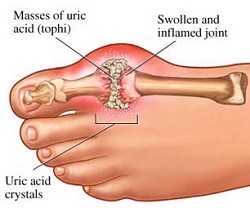
[[Image:Gout2.jpg|frame|right|A figure portraying the build up of uric acid in a synovial joint. Note that gout is most commonly presented in the toes or fingers.]]<span style="font-size: 13.28px;" /> | |||
nucleotide. In the case of GTP formation, IMP is first converted into [[XMP|XMP]] by IMP [[Dehydrogenase|dehydrogenase]]. This allows for the action of GTP synthase which rapidly converts XMP into GMP<ref>M W King. The Medical Biochemistry Page. September 2016. Cited: 11:31. 03.12.2016. Available from: http://themedicalbiochemistrypage.org/nucleotide-metabolism.php#purine</ref>. GMP is then phosphorylated by nucleoside phosphate kinases to yield diphosphates and eventually triphosphates. | |||
GTP is catabolised into insoluble [[Uric acid|uric acid]] which is present in the [[Urine|urine]] as [[Sodium urate|sodium urate crystals]]<ref>M W King. The Medical Biochemistry Page. September 2016. Cited: 11:31. 03.12.2016. Available from: http://themedicalbiochemistrypage.org/nucleotide-metabolism.php#purine</ref>. [[Gout|Gout is]] a manifestation of the abnormal [[Catabolism|catabolism]] of purine triphosphates in the [[Synovial joints|synovial joints]]. This leads to the presence of monosodium urate or calcium pyrophosphate dihydrate which causes clinical symptoms of [[Inflammation|inflammation]] and [[Arthritis|arthritis]]<ref>M W King. The Medical Biochemistry Page. September 2016. Cited: 11:31. 03.12.2016. Available from: http://themedicalbiochemistrypage.org/nucleotide-metabolism.php#purine</ref>. Severe combined immunodeficieny disease also can occur as a result of abnormal catabolism of purine triphosphates, which results in the destruction of [[B lymphocytes|B]] and [[T lymphocytes|T lymphocytes]]<ref>M W King. The Medical Biochemistry Page. September 2016. Cited: 11:31. 03.12.2016. Available from: http://themedicalbiochemistrypage.org/nucleotide-metabolism.php#purine</ref>. <br> | GTP is catabolised into insoluble [[Uric acid|uric acid]] which is present in the [[Urine|urine]] as [[Sodium urate|sodium urate crystals]]<ref>M W King. The Medical Biochemistry Page. September 2016. Cited: 11:31. 03.12.2016. Available from: http://themedicalbiochemistrypage.org/nucleotide-metabolism.php#purine</ref>. [[Gout|Gout is]] a manifestation of the abnormal [[Catabolism|catabolism]] of purine triphosphates in the [[Synovial joints|synovial joints]]. This leads to the presence of monosodium urate or calcium pyrophosphate dihydrate which causes clinical symptoms of [[Inflammation|inflammation]] and [[Arthritis|arthritis]]<ref>M W King. The Medical Biochemistry Page. September 2016. Cited: 11:31. 03.12.2016. Available from: http://themedicalbiochemistrypage.org/nucleotide-metabolism.php#purine</ref>. Severe combined immunodeficieny disease also can occur as a result of abnormal catabolism of purine triphosphates, which results in the destruction of [[B lymphocytes|B]] and [[T lymphocytes|T lymphocytes]]<ref>M W King. The Medical Biochemistry Page. September 2016. Cited: 11:31. 03.12.2016. Available from: http://themedicalbiochemistrypage.org/nucleotide-metabolism.php#purine</ref>. <br> | ||
Guanosine triphosphate will result in the formation of a guanine base as a result of cleavage of two [[Anhydride|anhydride bonds]], releasing two [[Free phosphate|free phosphates]] as products. However, this reaction will (normally) only be catalysed by[[RNA polymerase|RNA polymerase]] | |||
<span style="background-color: initial; font-size: 17.5296px; font-weight: bold;">One Of Many RNA Base Predecesors</span> | |||
RNA is chemically distinct from DNA primarily as a result of the existance of a [[Deoxyribose|deoxyribose]] instead of a ribose sugar. Guanosine triphosphate is concerned with the production of the guanine base only in RNA<ref>J Dow, G Lindsay and J Morrison, Biochemistry: Molecules, Cells and the Body. 1st Edition. Wokingham, England. Addison-Wesley. 1996.</ref>. In DNA | |||
[[Image:RNAdiNucSyn.gif|border|right|Formation of an RNA strand through anhydride bond cleavage, and subsequent phosphodiester bond formation. Note the 2'OH group on the ribose monosaccharide.]]<span style="font-size: 13.28px;" /> | |||
[[Deoxyguanosine|deoxyguanosine triphosphates]] a | |||
re used instead, as they do not possess a [[2'OH|2'OH group]] which makes them prone to nucleophilic attacks which can result in the [[Hydrolysis|hydrolysis]] of the base from the rest of the [[Polynucleotide|polynucleotide]]. | |||
Guanosine triphosphate will result in the formation of a guanine base as a result of cleavage of two [[Anhydride|anhydride bonds]], releasing two [[Free phosphate|free phosphates]] as products. However, this reaction will (normally) only be catalysed by[[RNA polymerase|RNA polymerase]] i | |||
f the opposite base is a cytosine with which the guanosine triphosphate can form [[Hydrogen bond|hydrogen bonds]]. It should be noted that this occurs independantly from the action of RNA polymerase, which merely forms phosphodiester bonds between already aligned triphosphates. After catalysis, the molecule is part of a polynucleotide chain and is no longer known as GTP, but as the base guanine. <br> | |||
=== GTP; A Cousin of Universal ATP === | === GTP; A Cousin of Universal ATP === | ||
The underlying mechanisms of yielding energy from anhydride bond cleavage is practically the same in all triphosphates. The high energy status of triphosphates is achieved through three distinct mechanisms. The first is due to the repulsive forces on each of the phosphate groups. This contributes to a high instability of the bonded triphosphates and a high "desire" to achieve a lower [[Gibbs free energy|energy]] status<ref>J M Berg, J L Tymoczko, G J Gatto and R Stryer. Biochemistry. Eighth Edition. City and Country Unidentified. Freeman and Co. 2015.</ref>. The second is due to [[Resonance|resonance stabilisation]]. When GTP is converted into GDP the amount of possible positions for the | The underlying mechanisms of yielding energy from anhydride bond cleavage is practically the same in all triphosphates. The high energy status of triphosphates is achieved through three distinct mechanisms. The first is due to the repulsive forces | ||
[[Image:Tubulin.jpg|right|300x200px|A figure depicting the effect GTP has on the assembly and disassembly of microtubules. Do you think that the same method is used to translocate organelles in the cytoplasm?]] | |||
on each of the phosphate groups. This contributes to a high instability of the bonded triphosphates and a high "desire" to achieve a lower [[Gibbs free energy|energy]] status<ref>J M Berg, J L Tymoczko, G J Gatto and R Stryer. Biochemistry. Eighth Edition. City and Country Unidentified. Freeman and Co. 2015.</ref>. The second is due to | |||
[[Image:Elongation.jpg|left|300x200px|A figure depicting the involvement of GTP in eukaryotic elongation. Comparison to prokaryotic translation reveals an increased complexity. Note that only knowledge of prokaryotic elongation is expected at Stage 1.]]<span style="font-size: 13.28px;" /> | |||
[[Resonance|resonance stabilisation]]. When GTP is converted into GDP the amount of | |||
possible positions for the e | |||
xistant [[Electron|electron pairs]] increases, lending to a greater stability<ref>J M Berg, J L Tymoczko, G J Gatto and R Stryer. Biochemistry. Eighth Edition. City and Country Unidentified. Freeman and Co. 2015.</ref>. This argument is also upheld with conversion into GMP. The third is due to the unfavourable [[Entropy|entropic effect]] an increased amount of phosphates have on the conformation of [[Water|water molecules]] that surround the molecule<ref>J M Berg, J L Tymoczko, G J Gatto and R Stryer. Biochemistry. Eighth Edition. City and Country Unidentified. Freeman and Co. 2015.</ref>. | |||
<div> | |||
Since GTP concentration in the [[Cytoplasm|cytoplasm]] is significantly lower than that of ATP, it is used for specific functions for cell metabolic processes. | |||
Primarily it is utilised for [[Protein synthesis|protein synthesis]] when coupled with [[IF2|IF2]] during ribosomal [[Ribosome intiation|initiation]], [[Ef-TU|Ef-Tu]] and | |||
<div> | |||
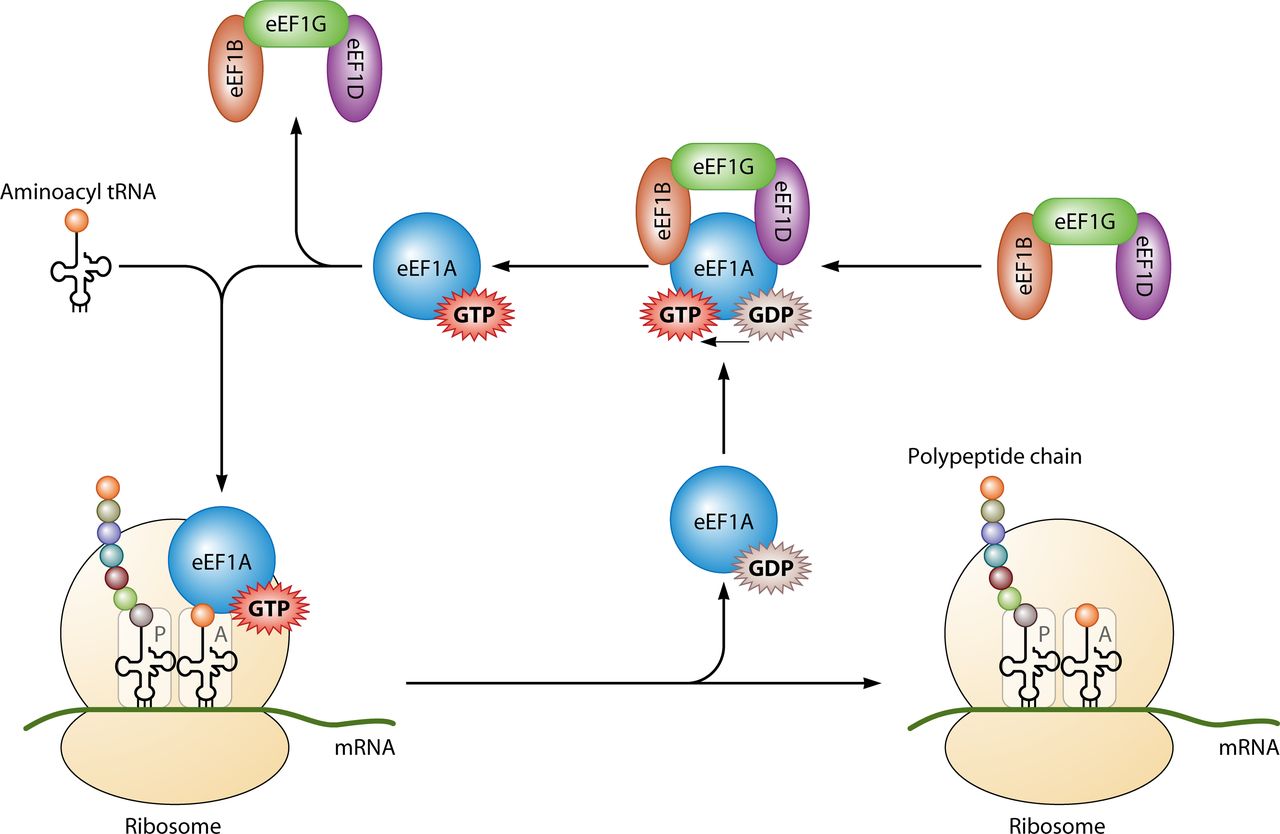
[[Ef-G|Ef-G during]] [[Elongation|elongation]] and [[RF3|RF3]] during [[Termination|termination]] (look left for the basic structure of the ribosomal complex) All of the GTP molecules that bind with the stated protein become hydrolysed in their distinct processes, releasing GDP and a free phosphate<ref>T E Dever and R Green. The Elongation, Termination and Recycling phases in Eukaryotes. CSHPB. July 2012. 4:7:1-16</ref>. | |||
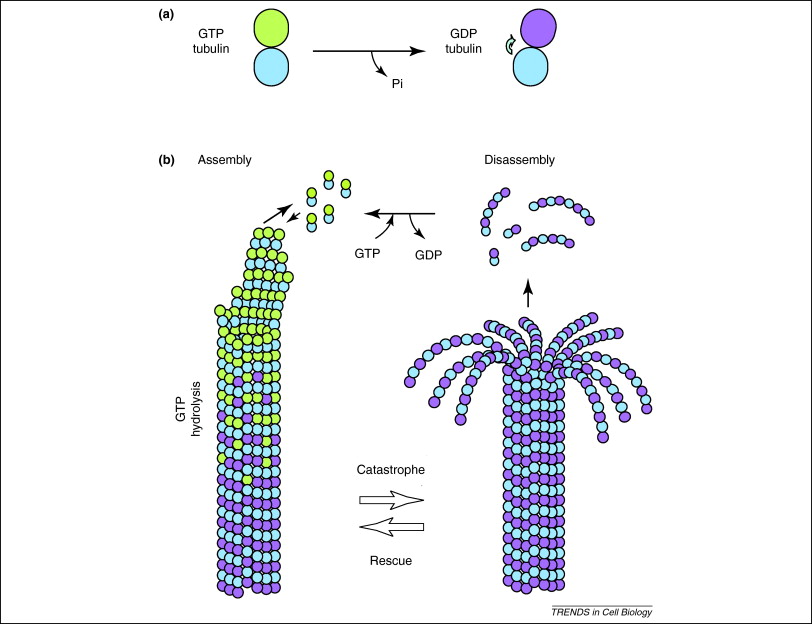
In addition, GTP is also utilised by [[Tubulin|tubulin]] dimers in the formation of [[Microtubules|microtubules.]] Tubulin dimers are composed of alpha and beta tubulin, each of which possess [[Cooperative binding|binding sites]] for GTP<ref>B Alberts et al. Molecular Biology of The Cell. 6th Edition. New York, USA. Garland Science. 2015.</ref>. Since beta tubulin exists at the plus end of the filament it is always hydrolysed when another dimer is added to the lengthening [[Polymer|polymer]]. The hydrolysis of GTP weakens the [[Non-covalent|non-covalent]] interactions between tubulin dimers are results in a facilitated ability to [[Dissolve|dissolve]] the microtubules when necessary<ref>B Alberts. Molecular Biology of The Cell. 6th Edition. New York, USA. Garland Science. 2015.</ref>. However, the alpha tubulin protein is not hydrolysed so it can be considered to be consistant throught the microtubule structure. | |||
=== A Resource for Signalling | === A Resource for Signalling === | ||
<br> | |||
[[Image:Heterog.png|frame|left|200x200px|An inactive heterotrimeric G protein. Which molecule does it have bound to it at this state?]] | |||
GTP is not only useful due to it's chemical properties, but also due to it's intricate structure. It is able to bind and regulate the activity of different signalling pathway proteins which are classified into two different groups. The first are [[G-protein|heterotrimeric GTP binding proteins]] (also known as heterotrimeric G proteins). These utilise GTP upon the activation of the [[G-protein linked receptor|G protein coupled receptor]] in order to affect other proteins in the pathway<ref>J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.</ref>. The second are [[Monomeric G-protein|monomeric GTP binding proteins]] (also known as monomeric G proteins). These respond to receptors other than G protein coupled receptors<ref>J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.</ref>. A typical example cited during their explanation is the [[Ras|Ras]] protein. They are able to selectively bind GTP not only due to it's specific chemistry but also due to the [[Induced fit mechanism|induced fit phenomenon]]. It is worth noting that the bound GTP will be hydrolysed after a certain period of time and thus result in an [[Auto-inactivation|auto-inactivatio]][[Auto-inactivation|n]] of the protein<ref>J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.</ref>. As a result they will thereafter contain a bound GDP molecule, but will uptake GTP upon a conformational change. GTP will be taken up after the [[Conformational change|conformational change]] due to a shift in [[Affinity|affinity]] towards the GTP molecule. | GTP is not only useful due to it's chemical properties, but also due to it's intricate structure. It is able to bind and regulate the activity of different signalling pathway proteins which are classified into two different groups. The first are [[G-protein|heterotrimeric GTP binding proteins]] (also known as heterotrimeric G proteins). These utilise GTP upon the activation of the [[G-protein linked receptor|G protein coupled receptor]] in order to affect other proteins in the pathway<ref>J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.</ref>. The second are [[Monomeric G-protein|monomeric GTP binding proteins]] (also known as monomeric G proteins). These respond to receptors other than G protein coupled receptors<ref>J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.</ref>. A typical example cited during their explanation is the [[Ras|Ras]] protein. They are able to selectively bind GTP not only due to it's specific chemistry but also due to the [[Induced fit mechanism|induced fit phenomenon]]. It is worth noting that the bound GTP will be hydrolysed after a certain period of time and thus result in an [[Auto-inactivation|auto-inactivatio]][[Auto-inactivation|n]] of the protein<ref>J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.</ref>. As a result they will thereafter contain a bound GDP molecule, but will uptake GTP upon a conformational change. GTP will be taken up after the [[Conformational change|conformational change]] due to a shift in [[Affinity|affinity]] towards the GTP molecule. | ||
GTP can also be used as a [[Reactant|reactant to]] produce cGMP which is a relatively common secondary signalling molecule. This catalysis is triggered by the release of NO which activates guanylate cyclase which consequently produces cGMP form GTP<ref>J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.</ref>. This secondary messenger can act as an effector towards protein kinases which phosphorylate and modify the action of specific proteins. | GTP can also be used as a [[Reactant|reactant to]] produce cGMP which is a relatively common secondary signalling molecule. This catalysis is triggered by the release of NO which activates guanylate cyclase which consequently produces cGMP form GTP<ref>J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.</ref>. This secondary messenger can act as an effector towards protein kinases which phosphorylate and modify the action of specific proteins. | ||
=== References === | === References === | ||
<references /><br> | <references /><br> | ||
</div> | |||
</div> | |||
Revision as of 08:39, 5 December 2016
Guanosine triphosphate (Guanosine-5'-triphosphate to be precise or also commonly abbreviated GTP for simplicity) is a high energy nucleotide (not to be confused with nucleoside).

As a result of it's structure it has selective roles in the formation of RNA strands[1], functioning as an energy carrier molecule for protein synthesis[2], a coenzyme, a predecessor to cGMP - a secondary messenger molecule[3] or as an effector molecule. The last two are commonly demonstrated by G-protein modulation[4]. It is important to note that this list does not exhuast it's chemical interactions but is merely a demonstration of it's various capabilities.
In GTP the ribose sugar is central to the three dimensional arrangement of the covalently bonded guanine and triphosphate molecules. This monosaccharide provide hydroxyl groups for condensation reactions and nucleophilic attacks[5].The guanine molecule and the triphosphate form covalent bonds at C'1 and C'5 atoms respectively. The purine is bonded as a result of a condensation reaction at it's 9'N. Since guanine is a purine base, it is classified as a purine triphosphate along with adenine triphosphate (ATP)[6]. It is formed along with ATP through inosine monophosphate modification[7]. It's structural formula (right) suggests it's chemical activity and is described further in detail below.
Misconception: GTP, A Baseline Building Block
nucleotide. In the case of GTP formation, IMP is first converted into XMP by IMP dehydrogenase. This allows for the action of GTP synthase which rapidly converts XMP into GMP[9]. GMP is then phosphorylated by nucleoside phosphate kinases to yield diphosphates and eventually triphosphates.
GTP is catabolised into insoluble uric acid which is present in the urine as sodium urate crystals[10]. Gout is a manifestation of the abnormal catabolism of purine triphosphates in the synovial joints. This leads to the presence of monosodium urate or calcium pyrophosphate dihydrate which causes clinical symptoms of inflammation and arthritis[11]. Severe combined immunodeficieny disease also can occur as a result of abnormal catabolism of purine triphosphates, which results in the destruction of B and T lymphocytes[12].
One Of Many RNA Base Predecesors
RNA is chemically distinct from DNA primarily as a result of the existance of a deoxyribose instead of a ribose sugar. Guanosine triphosphate is concerned with the production of the guanine base only in RNA[13]. In DNA
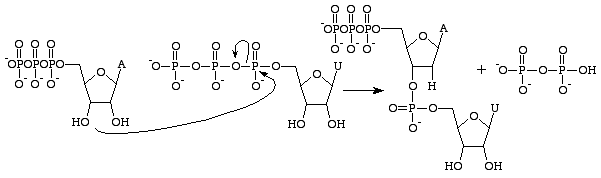
deoxyguanosine triphosphates a
re used instead, as they do not possess a 2'OH group which makes them prone to nucleophilic attacks which can result in the hydrolysis of the base from the rest of the polynucleotide.
Guanosine triphosphate will result in the formation of a guanine base as a result of cleavage of two anhydride bonds, releasing two free phosphates as products. However, this reaction will (normally) only be catalysed byRNA polymerase i
f the opposite base is a cytosine with which the guanosine triphosphate can form hydrogen bonds. It should be noted that this occurs independantly from the action of RNA polymerase, which merely forms phosphodiester bonds between already aligned triphosphates. After catalysis, the molecule is part of a polynucleotide chain and is no longer known as GTP, but as the base guanine.
GTP; A Cousin of Universal ATP
The underlying mechanisms of yielding energy from anhydride bond cleavage is practically the same in all triphosphates. The high energy status of triphosphates is achieved through three distinct mechanisms. The first is due to the repulsive forces
on each of the phosphate groups. This contributes to a high instability of the bonded triphosphates and a high "desire" to achieve a lower energy status[14]. The second is due to
resonance stabilisation. When GTP is converted into GDP the amount of
possible positions for the e
xistant electron pairs increases, lending to a greater stability[15]. This argument is also upheld with conversion into GMP. The third is due to the unfavourable entropic effect an increased amount of phosphates have on the conformation of water molecules that surround the molecule[16].
Since GTP concentration in the cytoplasm is significantly lower than that of ATP, it is used for specific functions for cell metabolic processes.
Primarily it is utilised for protein synthesis when coupled with IF2 during ribosomal initiation, Ef-Tu and
Ef-G during elongation and RF3 during termination (look left for the basic structure of the ribosomal complex) All of the GTP molecules that bind with the stated protein become hydrolysed in their distinct processes, releasing GDP and a free phosphate[17].
In addition, GTP is also utilised by tubulin dimers in the formation of microtubules. Tubulin dimers are composed of alpha and beta tubulin, each of which possess binding sites for GTP[18]. Since beta tubulin exists at the plus end of the filament it is always hydrolysed when another dimer is added to the lengthening polymer. The hydrolysis of GTP weakens the non-covalent interactions between tubulin dimers are results in a facilitated ability to dissolve the microtubules when necessary[19]. However, the alpha tubulin protein is not hydrolysed so it can be considered to be consistant throught the microtubule structure.
A Resource for Signalling
GTP is not only useful due to it's chemical properties, but also due to it's intricate structure. It is able to bind and regulate the activity of different signalling pathway proteins which are classified into two different groups. The first are heterotrimeric GTP binding proteins (also known as heterotrimeric G proteins). These utilise GTP upon the activation of the G protein coupled receptor in order to affect other proteins in the pathway[20]. The second are monomeric GTP binding proteins (also known as monomeric G proteins). These respond to receptors other than G protein coupled receptors[21]. A typical example cited during their explanation is the Ras protein. They are able to selectively bind GTP not only due to it's specific chemistry but also due to the induced fit phenomenon. It is worth noting that the bound GTP will be hydrolysed after a certain period of time and thus result in an auto-inactivation of the protein[22]. As a result they will thereafter contain a bound GDP molecule, but will uptake GTP upon a conformational change. GTP will be taken up after the conformational change due to a shift in affinity towards the GTP molecule.
GTP can also be used as a reactant to produce cGMP which is a relatively common secondary signalling molecule. This catalysis is triggered by the release of NO which activates guanylate cyclase which consequently produces cGMP form GTP[23]. This secondary messenger can act as an effector towards protein kinases which phosphorylate and modify the action of specific proteins.
References
- ↑ J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.
- ↑ R K Murray, D A Bender, K M Botham, P J Kennelly, V W Rodwell and P A Weil. Harper's Illustrated Biochemistry. 28th Edition. Beijing, China. 2009.
- ↑ J Dow, G Lindsay and J Morrison, Biochemistry: Molecules, Cells and the Body. 1st Edition. Wokingham, England. Addison-Wesley. 1996.
- ↑ J Dow, G Lindsay and J Morrison, Biochemistry: Molecules, Cells and the Body. 1st Edition. Wokingham, England. Addison-Wesley. 1996.
- ↑ J Dow, G Lindsay and J Morrison, Biochemistry: Molecules, Cells and the Body. 1st Edition. Wokingham, England. Addison-Wesley. 1996.
- ↑ J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.
- ↑ J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.
- ↑ M W King. The Medical Biochemistry Page. September 2016. Cited: 11:31. 03.12.2016. Available from: http://themedicalbiochemistrypage.org/nucleotide-metabolism.php#purine
- ↑ M W King. The Medical Biochemistry Page. September 2016. Cited: 11:31. 03.12.2016. Available from: http://themedicalbiochemistrypage.org/nucleotide-metabolism.php#purine
- ↑ M W King. The Medical Biochemistry Page. September 2016. Cited: 11:31. 03.12.2016. Available from: http://themedicalbiochemistrypage.org/nucleotide-metabolism.php#purine
- ↑ M W King. The Medical Biochemistry Page. September 2016. Cited: 11:31. 03.12.2016. Available from: http://themedicalbiochemistrypage.org/nucleotide-metabolism.php#purine
- ↑ M W King. The Medical Biochemistry Page. September 2016. Cited: 11:31. 03.12.2016. Available from: http://themedicalbiochemistrypage.org/nucleotide-metabolism.php#purine
- ↑ J Dow, G Lindsay and J Morrison, Biochemistry: Molecules, Cells and the Body. 1st Edition. Wokingham, England. Addison-Wesley. 1996.
- ↑ J M Berg, J L Tymoczko, G J Gatto and R Stryer. Biochemistry. Eighth Edition. City and Country Unidentified. Freeman and Co. 2015.
- ↑ J M Berg, J L Tymoczko, G J Gatto and R Stryer. Biochemistry. Eighth Edition. City and Country Unidentified. Freeman and Co. 2015.
- ↑ J M Berg, J L Tymoczko, G J Gatto and R Stryer. Biochemistry. Eighth Edition. City and Country Unidentified. Freeman and Co. 2015.
- ↑ T E Dever and R Green. The Elongation, Termination and Recycling phases in Eukaryotes. CSHPB. July 2012. 4:7:1-16
- ↑ B Alberts et al. Molecular Biology of The Cell. 6th Edition. New York, USA. Garland Science. 2015.
- ↑ B Alberts. Molecular Biology of The Cell. 6th Edition. New York, USA. Garland Science. 2015.
- ↑ J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.
- ↑ J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.
- ↑ J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.
- ↑ J Koolman and KH Roehm, Color Atlas of Biochemistry, 3rd Edition, Stuttgart, Germany. Thieme 2013.