Phosphodiester bond: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
The phosphodiester bond links two [[Pentose|pentose ]](5 carbon sugar) to a [[Phosphate group|phosphate group]] by strong, covalent, ester bonds. The formation of these bonds is a [[Condensation Reaction|condensation reaction]] in which water is lost. This bond is a key structural feature of the backbone of DNA and RNA and links the 3’ carbon of one nucleotide to the 5’ carbon of another to produce the strands of [[DNA|DNA ]] | The phosphodiester bond links two [[Pentose|pentose ]](5 carbon sugar) to a [[Phosphate group|phosphate group]] by strong, [[covalent|covalent]], [[ester bond|ester bonds]]. The formation of these bonds is a [[Condensation Reaction|condensation reaction]] in which water is lost. This bond is a key structural feature of the backbone of DNA and RNA and links the 3’ carbon of one nucleotide to the 5’ carbon of another to produce the strands of [[DNA|DNA and]] [[RNA|RNA]]. | ||
= Phosphodiester Bond Formation = | = Phosphodiester Bond Formation = | ||
| Line 13: | Line 13: | ||
= <br>Phosphodiester Bond Hydrolysis = | = <br>Phosphodiester Bond Hydrolysis = | ||
In phosphodiester [[Hydrolysis|hydrolysis]], water dissociated into H+ and OH-. The OH- acts as the [[Nucleophile|nucleophile in]] the [[Nucleophilic substitution|nucleophilic substitution]] reaction of hydrolysis. The reaction is catalysed by [[Phosphodiesterase|phosphodiesterase]].<br>The mechanism of this reaction is given below. | In phosphodiester [[Hydrolysis|hydrolysis]], water dissociated into H<sup><sub>+</sub></sup>and OH<sup>-</sup>. The OH<sup>-</sup> acts as the [[Nucleophile|nucleophile in]] the [[Nucleophilic substitution|nucleophilic substitution]] reaction of hydrolysis. The reaction is catalysed by [[Phosphodiesterase|phosphodiesterase]].<br>The mechanism of this reaction is given below. | ||
[[Image:Phosphodiester hydrolysis mechanism.png|992x208px]]<br><br> | [[Image:Phosphodiester hydrolysis mechanism.png|992x208px]]<br><br> | ||
Revision as of 16:04, 12 December 2010
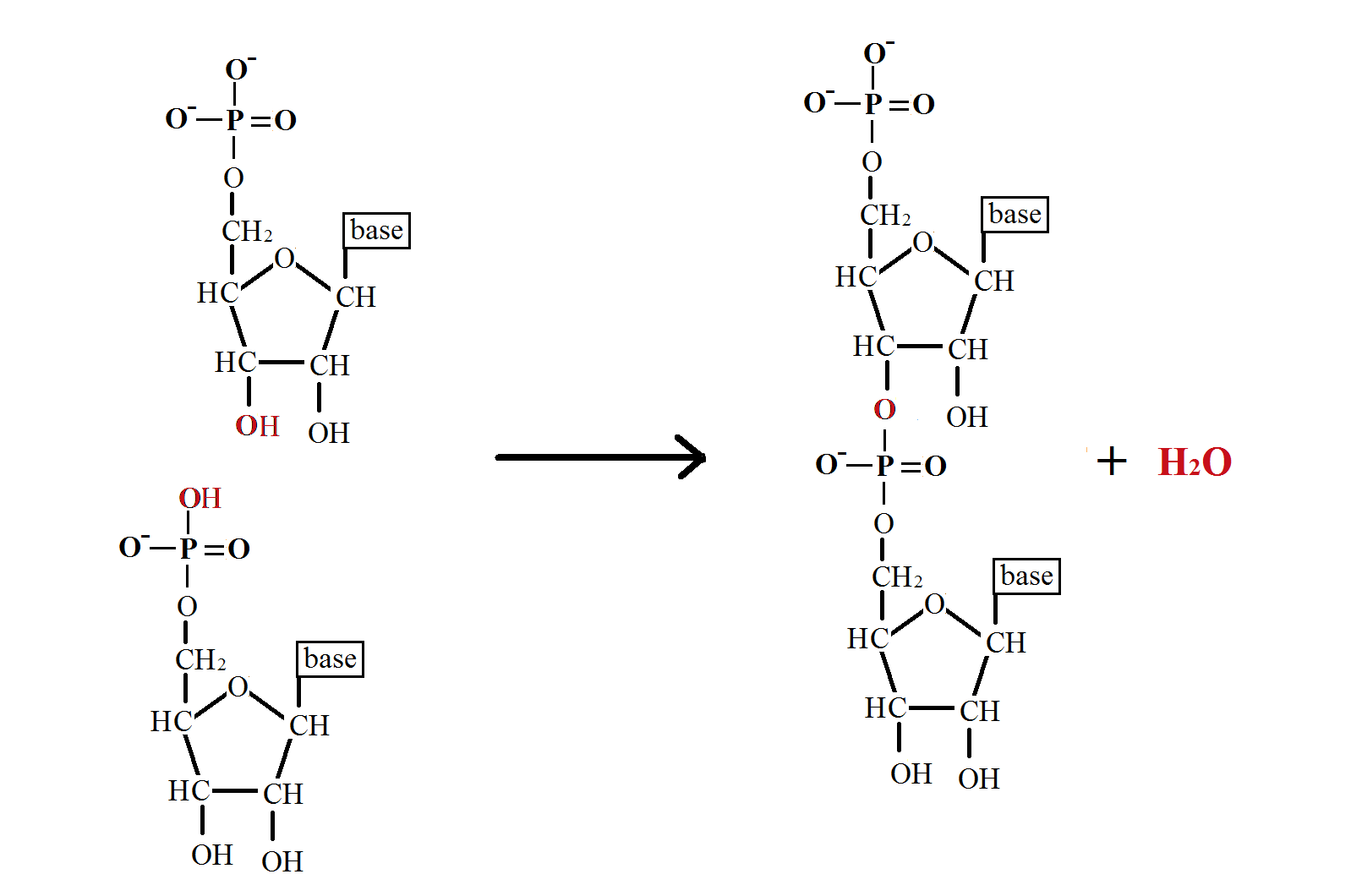
The phosphodiester bond links two pentose (5 carbon sugar) to a phosphate group by strong, covalent, ester bonds. The formation of these bonds is a condensation reaction in which water is lost. This bond is a key structural feature of the backbone of DNA and RNA and links the 3’ carbon of one nucleotide to the 5’ carbon of another to produce the strands of DNA and RNA.
Phosphodiester Bond Formation
In phosphodiester formation, the 1’ and 2’ hydroxyl (OH) groups of the phosphate molecule bind to the 3’ and 5’ carbons of the two independent pentose sugars. These are two condensation reactions so produce two molecules of water. The phosphate is then bonded to the sugars by two ester bonds, so is called a phosphodiester bond. This reaction is catalysed by ligases, such as DNA ligase during DNA replication.
The mechanism for this reaction is given below.
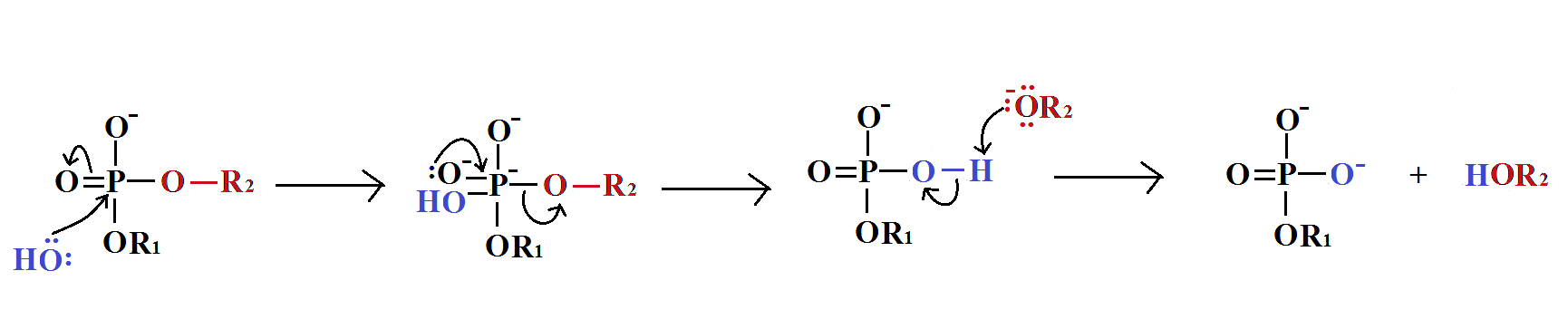
Phosphodiester Bond Hydrolysis
In phosphodiester hydrolysis, water dissociated into H+and OH-. The OH- acts as the nucleophile in the nucleophilic substitution reaction of hydrolysis. The reaction is catalysed by phosphodiesterase.
The mechanism of this reaction is given below.