Krebs cycle
Introduction
The Krebs Cycle can also be called the Citric Acid Cycle (CAC) or the Tricarboxylic Acid (TCA) Cycle. This cycle takes place in the Mitochondrial matrix and is the primary step of aerobic processing within a cell. The process oxidises glucose derivatives, fatty acids and amino acids to carbon dioxide (CO2) through a series of enzyme controlled steps. The purpose of the Krebs Cycle is to collect (eight) high-energy electrons from these fuels by oxidising them, which are transported by activated carriers NADH and FADH2 to the electron transport chain. The Krebs Cycle is also the source for the precursors of many other molecules, and is therefore an amphibolic pathway (anabolic and catabolic) [1][2].
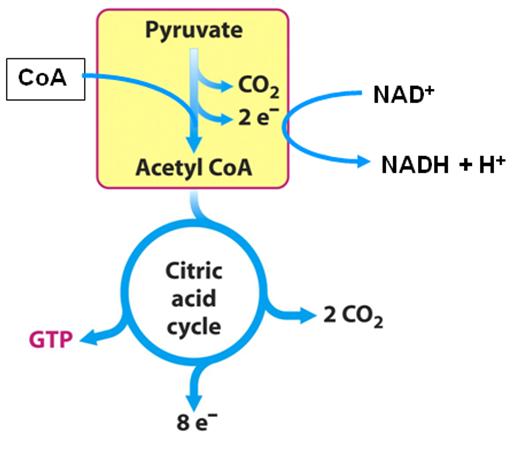
The 8 Steps of the Krebs Cycle
Step 0
* Not actually part of the Krebs Cycle. This step simply links glycolysis to the Krebs Cycle.
Glycolysis produces pyruvate which, under aerobic conditions, gets moved into the mitochondria via a carrier protein within the membrane. There it is oxidatively decarboxylated by a huge enzyme complex called the pyruvate dehydrogenase complex. This reaction is irreversible and requires coenzyme A as well as producing 1 CO2 and picks up two electrons by NAD+ [3].
| Reactants | Enzyme | Products | Reaction type | (Ir)reversible |
| Pyruvate(3C) + CoA(1C) + NAD+ | pyruvate dehydrogenase | Acetyl CoA(2C) + CO2 + NADH + H+ | Oxidative decarboxylation | Irreversible |
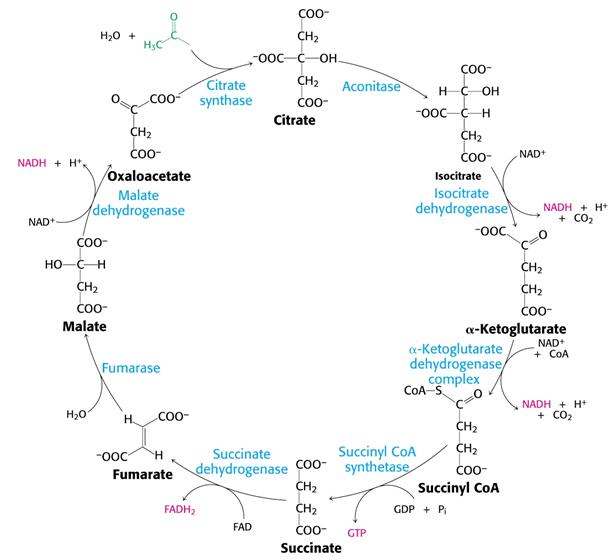
Step 1
| Reactants | Enzyme | Products | Reaction type | (Ir)reversible |
| Acetyl CoA (2C) + Oxaloacetate (4C) + H20 | Citrate synthase | Citrate (6C) + CoA + H+ | Aldol condensation and hydrolysis | Irreversible |
The oxaloacetate and acetyl CoA are first condensed forming citryl CoA which is then hydrolysed to CoA and citrate. This second step gives the power for the whole reaction to move forward and make citrate. The oxaloacetate binds to the citrate synthase enzyme first and causes a big conformational change which causes it to make the acetyl CoA binding site by altering the enzyme from the open to the closed form. The enzyme performs the condensation by getting both substrates close together, positioning them appropriately and then polarising specific bonds. When citryl CoA is formed, it causes the enzyme’s active site to close fully [4].
Step 2
| Reactants | Enzyme | Products | Reaction type | (Ir)reversible |
| Citrate (6C) | Aconitase | Isocitrate (6C) | Reshuffling (dehydrating and then hydrating) | Reversible |
This step is required to relocate the hydroxyl group on the citrate molecule so that oxidative decarboxylation can take place. The enzyme aconitase contains iron that is not bonded to a heme which means it is an iron-sulphur protein and this 4Fe-S cluster is what takes part in the dehydrating and hydrating of citrate [5].
Step 3
| Reactants | Enzyme | Products | Reaction type | (Ir)reversible |
| Isocitrate (6C) + NAD+ | Isocitrate dehydrogenase | α-Ketoglutarate (5C) + CO2 + NADH | Oxidative decarboxylation | Reversible |
Oxalosuccinate is an unstable β-ketoacid and is the intermediate of the reaction in step 3. How quickly the α-Ketoglutarate is produced determines the rate of the whole cycle [6].
Step 4
| Reactants | Enzyme | Products | Reaction type | (Ir)reversible |
| α-Ketoglutarate (5C) + NAD+ + CoA | α-Ketoglutarate dehydrogenase complex | Succinyl CoA (4C) + CO2 + NADH | Oxidative decarboxylation | Reversible |
The α-ketoglutarate dehydrogenase complex is a group of three kinds of enzymes and is homologous to the pyruvate dehydrogenase complex in glycolysis. Their oxidative decarboxylation process is similar because pyruvate and α-ketoglutarate are both α-ketoacid are the process has analogous reaction mechanisms [7].
Step 5
| Reactants | Enzyme | Products | Reaction type | (Ir)reversible |
| Succinyl CoA (4C) + Pi + GDP + H20 | Succinyl CoA synthase | Succinate (4C) + GTP + CoA | Substrate-level phosphorylation | Reversible |
The phosphorylation of GDP to GTP is coupled by the breakdown of the thioester bond in the molecule of succinyl CoA which powers it.[8]
Step 6
| Reactants | Enzyme | Products | Reaction type | (Ir)reversible |
| Succinate (4C) + FAD | Succinnate dehydrogenase | Fumerate (4C) + FADH2 | Oxidation | Reversible |
This is where the regeneration of oxaloacetate, the last stage, in the Krebs cycle begins.
The hydrogen acceptor in this step is FAD instead of NAD because the free energy change, ΔG, due to the oxidation is only high enough to be able to reduce FAD and not enough to reduce NAD+.
Succinnate dehydrogenase is also an iron-sulphur protein just like aconitase but is imbedded in the inner membrane of the mitochondria and is therefore the direct link between the Krebs cycle and the electron transport chain. ===== ===== The FADH2 donates its two electrons to the Fe-S clusters in the enzyme which then transfers them onto coenzyme Q (CoQ) that passes through the electron transport chain [9].
Step 7
| Reactants | Enzyme | Products | Reaction type | (Ir)reversible |
| Fumerate (4C) + H20 | Fumerase | L- Malate (4C) | Hydration | Reversible |
The reason why only the L form of the malate is formed is because he enzyme fumerase is stereospecific and only adds H+ and OH- to one side of the double bond in fumarate [10].
Step 8
| Reactants | Enzyme | Products | Reaction type | (Ir)reversible |
| L-Malate (4C) + NAD+ | Malate dehydrogenase | Oxaloacetate (4C) + NAD + H+ | Oxidation | Reversible |
This oxidation reaction is moved along by the use of oxaloacetate and NADH [11][12].
Products of the Krebs Cycle
Overall:
Acetyl CoA + 3NAD+ + FAD + GDP + Pi + H20
gives
-> 2CO2 + 3NADH + FADH2 + GTP + CoA + 2H+
(Remember: multiple these by 2 because for every 1 glucose we get 2 pyruvate molecules and thus 2 acetyl CoA molecules) [13]
Control of the Krebs Cycle
The Krebs Cycle is inhibited by high energy levels and encouraged by low energy levels in the cell.
There are 3 control points in the Krebs Cycle:
1. Pyruvate dehydrogenase
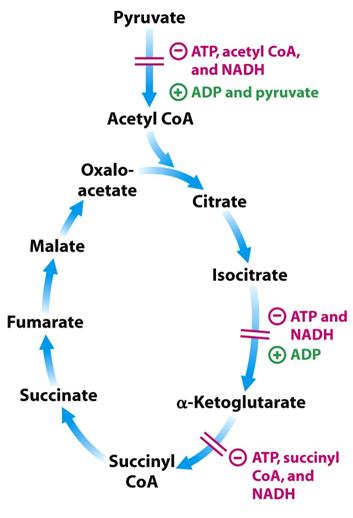
Encouraged by high levels of: ADP and pyruvate
Inhibited by high levels of: ATP, acetyl CoA and NADH
Acetyl CoA inhibits the transacetylase component, E2, by binding to it directly.
NADH on the other hand inhibits the dihydrolipoyl dehydrogenase E3.
The way in which this enzyme complex is regulated is through covalent modification by phosphorylation. To switch off the activity of the enzyme a specific kinase will phosphorylate the enzyme complex and to reactivate it a specific phophotase is needed.
2. Isocitrate dehydrogenase
Inhibited by high levels of: ATP and NADH
Encouraged by high levels of: ADP
ADP allosterically encourages the enzyme by increasing its affinity for isocitrate.
3. α-Ketoglutarate dehydrogenase
Inhibited by high levels of: ATP, succinyl CoA and NADH [14]
Anabolic Properties of the Krebs Cycle
Citrate -> sterols and fatty acids
α-Ketoglutarate -> glutamate, other amino acids and purines
Succinyl CoA -> porphyrins, heme and chlorophyll
Oxaloacetate -> aspartate, other amino acids, purines and pyrimidines [15]

References
- ↑ Berg J.M, Tymoczko J.L, Stryer, L (2007). Biochemistry. 6th ed. New York: W. H. Freeman and Company. p475-476
- ↑ Alberts, B. Johnson, A. Lewis, J. Raff, M. Roberts, K. and Walter, P. (2008). Molecular Biology of The Cell. 5th ed. New York and Abingdon: Garland Science. p97-98
- ↑ Berg J.M, Tymoczko J.L, Stryer, L (2007). Biochemistry. 6th ed. New York: W. H. Freeman and Company. p477
- ↑ Berg J.M, Tymoczko J.L, Stryer, L (2007). Biochemistry. 6th ed. New York: W. H. Freeman and Company. p482-483
- ↑ Berg J.M, Tymoczko J.L, Stryer, L (2007). Biochemistry. 6th ed. New York: W. H. Freeman and Company. p484
- ↑ Berg J.M, Tymoczko J.L, Stryer, L (2007). Biochemistry. 6th ed. New York: W. H. Freeman and Company. p484-485
- ↑ Berg J.M, Tymoczko J.L, Stryer, L (2007). Biochemistry. 6th ed. New York: W. H. Freeman and Company. p485
- ↑ Berg J.M, Tymoczko J.L, Stryer, L (2007). Biochemistry. 6th ed. New York: W. H. Freeman and Company. p485-486
- ↑ Berg J.M, Tymoczko J.L, Stryer, L (2007). Biochemistry. 6th ed. New York: W. H. Freeman and Company. p486
- ↑ Berg J.M, Tymoczko J.L, Stryer, L (2007). Biochemistry. 6th ed. New York: W. H. Freeman and Company. p487-488
- ↑ Berg J.M, Tymoczko J.L, Stryer, L (2007). Biochemistry. 6th ed. New York: W. H. Freeman and Company. p488
- ↑ Berg J.M, Tymoczko J.L, Stryer, L (2007). Biochemistry. 6th ed. New York: W. H. Freeman and Company. p489
- ↑ Berg J.M, Tymoczko J.L, Stryer, L (2007). Biochemistry. 6th ed. New York: W. H. Freeman and Company. p488
- ↑ Berg J.M, Tymoczko J.L, Stryer, L (2007). Biochemistry. 6th ed. New York: W. H. Freeman and Company. p490-493
- ↑ Alberts, B. Johnson, A. Lewis, J. Raff, M. Roberts, K. and Walter, P. (2008). Molecular Biology of The Cell. 5th ed. New York and Abingdon: Garland Science. p99